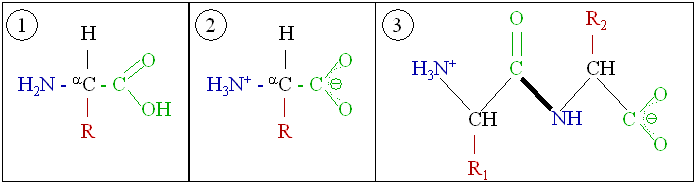
Amino acid
|
|
In chemistry, an amino acid is any molecule that contains both amino and carboxylic acid functional groups. In biochemistry, this shorter and more general term is frequently used to refer to alpha amino acids: those amino acids in which the amino and carboxylate functionalities are attached to the same carbon.
Amino acid residue is what is left of an amino acid once a water molecule has been lost (an H+ from the nitrogenous side and an OH- from the carboxylic side) in the formation of a peptide bond .
| Contents |
Overview
Amino acids are the basic structural building units of proteins. They form short polymer chains called peptides or polypeptides which in turn form structures called proteins.
Twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. Rarer, more complicated ones are produced by the body and are called nonstandard. Proline is the only proteinogenic amino acid whose side group is cyclic and links to the a-amino group, forming a secondary amino group. Formerly, proline was misleadingly called an imino acid. Other amino acids contained in proteins are usually formed by post-translational modification, that is modification after translation (protein synthesis). These modifications are often essential for the function of the protein. At least two amino acids other than the standard 20 are sometimes incorporated into proteins during translation:
- Selenocysteine is incorporated into some proteins at a UGA codon, which is normally a stop codon.
- Pyrrolysine is used by some methanogens in enzymes that they use to produce methane. It is coded for similarity to selenocysteine but with the codon UAG instead.
Although only 20 amino acids are genetically coded, over 100 have been found in nature. Some of these have been detected in meteorites, especially in a type known as carbonaceous chondrites. Microorganisms and plants often produce very uncommon amino acids, which can be found in peptidic antibiotics (e.g. nisin or alamethicin). Lanthionine is a sulfide-bridged alanine dimer which is found together with unsaturated amino acids in lantibiotics (antibiotic peptides of microbial origin). 1-Aminocyclopropane-1-carboxylic acid (ACC) is a small disubstituted cyclic amino acid and a key intermediate in the production of the plant hormone ethylene.
In addition to amino acids for protein synthesis, there are other biologically important amino acids, such as the neurotransmitters glycine, GABA and glutamate, as well as carnitine (used in lipid transport within a cell), ornithine, citrulline, homocysteine, hydroxyproline, hydroxylysine, and sarcosine.
Some of the 20 standard amino acids are called essential amino acids, because they cannot be synthesized by the body from other compounds through chemical reactions, but instead must be taken in with food. In humans, the essential amino acids are lysine, leucine, isoleucine, methionine, phenylalanine, threonine, tryptophan, valine, and (in children) histidine and arginine.
General structure
The general structure of proteinogenic alpha amino acids is:
COOH | H-C-R | NH2
Where "R" represents a side chain specific to each amino acid. Amino acids are usually classified by properties of the side chain into four groups: acidic, basic, hydrophilic (polar), and hydrophobic (nonpolar).
Isomerism
Except for glycine, where R = H, amino acids occur in two possible optical isomers, called D and L. L amino acids represent the vast majority of amino acids found in proteins. D amino acids are found in some proteins produced by exotic sea-dwelling organisms, such as cone snails. They are also abundant components of the cell walls of bacteria.
Reactions
Proteins are created by polymerization of amino acids by peptide bonds in a process called translation.
1. Amino acid; 2, zwitterion structure; 3, two amino acids forming a peptide bond. (See also bond.)
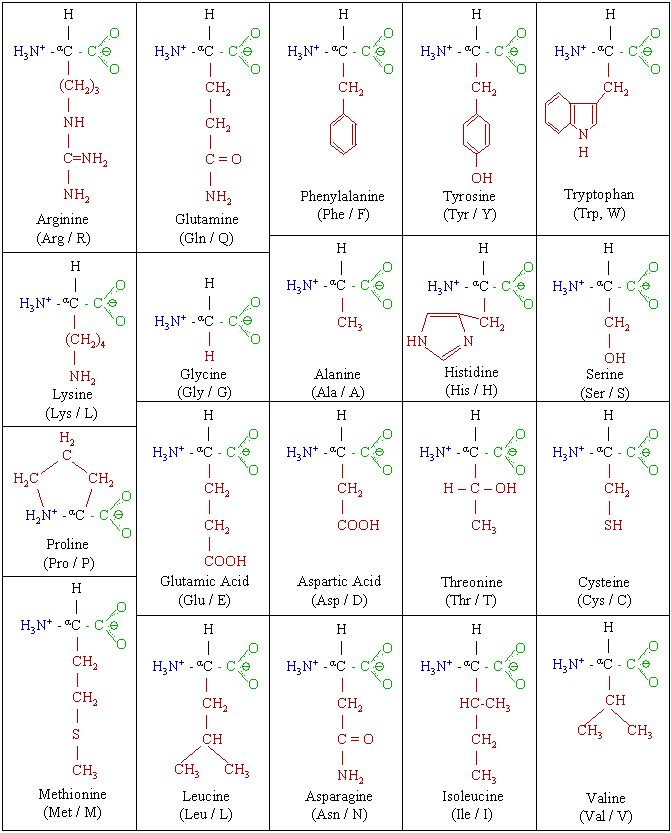
List of standard amino acids
Structures
Structures and symbols of the 20 amino acids present in genetic code.
Chemical properties
Following is a table listing the one letter symbols, the three letter symbols, and the chemical properties of the side chains of the standard amino acids. The one letter symbol for an undetermined amino acid is X. The three letter symbol asx means the amino acid is either asparagine or aspartic acid.
| Abbrev. | Full Name | Side chain type | Mass | pI | pK1 (α-COOH) | pK2 (α-+NH3) | pKr (R) | Remarks | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A | Ala | Alanine | hydrophobic | 89.09 | 6.11 | 2.35 | 9.87 | |||
| C | Cys | Cysteine | hydrophobic (Nagano, 1999) | 121.16 | 5.05 | 1.92 | 10.70 | 8.37 | Under oxidizing conditions, two cysteines can join together by a disulfide bond to form the amino acid cystine. When cysteines are part of a protein, insulin for example, this enforces tertiary structure. | |
| D | Asp <td nowrap>Aspartic acid | acidic | 133.10 | 2.85 | 1.99 | 9.90 | 3.90 | |||
| E | Glu <td nowrap>Glutamic acid | acidic | 147.13 | 3.15 | 2.10 | 9.47 | 4.07 | |||
| F | Phe | Phenylalanine | hydrophobic | 165.19 | 5.49 | 2.20 | 9.31 | |||
| G | Gly | Glycine | hydrophilic | 75.07 | 6.06 | 2.35 | 9.78 | Because of the two hydrogen atoms at the α carbon, glycine is not optically active. | ||
| H | His | Histidine | basic | 155.16 | 7.60 | 1.80 | 9.33 | 6.04 | In even slightly acidic conditions protonation of the nitrogen occurs, changing the properties of histidine and the polypeptide as a whole. It is used by many proteins as a regulatory mechanism, changing the conformation and behavior of the polypeptide in acidic regions such as the late endosome or lysosome. | |
| I | Ile | Isoleucine | hydrophobic | 131.17 | 6.05 | 2.32 | 9.76 | |||
| K | Lys | Lysine | basic | 146.19 | 9.60 | 2.16 | 9.06 | 10.54 | ||
| L | Leu | Leucine | hydrophobic | 131.17 | 6.01 | 2.33 | 9.74 | |||
| M | Met | Methionine | hydrophobic | 149.21 | 5.74 | 2.13 | 9.28 | Always the first amino acid to be incorporated into a protein; sometimes removed after translation. | ||
| N | Asn | Asparagine | hydrophilic | 132.12 | 5.41 | 2.14 | 8.72 | |||
| P | Pro | Proline | hydrophobic | 115.13 | 6.30 | 1.95 | 10.64 | Can disrupt protein folding structures like α helix or β sheet. | ||
| Q | Gln | Glutamine | hydrophilic | 146.15 | 5.65 | 2.17 | 9.13 | |||
| R | Arg | Arginine | basic | 174.20 | 10.76 | 1.82 | 8.99 | 12.48 | ||
| S | Ser | Serine | hydrophilic | 105.09 | 5.68 | 2.19 | 9.21 | |||
| T | Thr | Threonine | hydrophilic | 119.12 | 5.60 | 2.09 | 9.10 | |||
| V | Val | Valine | hydrophobic | 117.15 | 6.00 | 2.39 | 9.74 | |||
| W | Trp | Tryptophan | hydrophobic | 204.23 | 5.89 | 2.46 | 9.41 | |||
| Y | Tyr | Tyrosine | hydrophobic | 181.19 | 5.64 | 2.20 | 9.21 | 10.46 | ||
| Amino acid | Hydrophobic | Positive | Negative | Polar | Charged | Small | Tiny | Aromatic | Aliphatic | van der Waals volume | Codon | Occurrence in proteins (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alanine | X | - | - | - | - | X | X | - | - | 67 | GCU, GCC, GCA, GCG | 7.8 |
| Cysteine | X | - | - | - | - | X | - | - | - | 86 | UGU, UGC | 1.9 |
| Aspartate | - | - | X | X | X | X | - | - | - | 91 | GAU, GAC | 5.3 |
| Glutamate | - | - | X | X | X | - | - | - | - | 109 | GAA, GAG | 6.3 |
| Phenylalanine | X | - | - | - | - | - | - | X | - | 135 | UUU, UUC | 3.9 |
| Glycine | X | - | - | - | - | X | X | - | - | 48 | GGU, GGC, GGA, GGG | 7.2 |
| Histidine | X | X | - | X | X | - | - | X | - | 118 | CAU, CAC | 2.3 |
| Lysine | - | X | - | X | X | - | - | - | - | 135 | AAA, AAG | 5.9 |
| Isoleucine | X | - | - | - | - | - | - | - | X | 124 | AUU, AUC, AUA | 5.3 |
| Leucine | X | - | - | - | - | - | - | - | X | 124 | UUA, UUG, CUU, CUC, CUA, CUG | 9.1 |
| Methionine | X | - | - | - | - | - | - | - | - | 124 | AUG | 2.3 |
| Asparagine | - | - | - | X | - | X | - | - | - | 96 | AAU, AAC | 4.3 |
| Proline | - | - | - | - | - | X | - | - | - | 90 | CCU, CCC, CCA, CCG | 5.2 |
| Glutamine | - | - | - | X | - | - | - | - | - | 114 | GGU, GGC, GGA, GGG | 4.2 |
| Arginine | - | X | - | X | X | - | - | - | - | 148 | CGU, CGC, CGA, CGG, AGA, AGG | 5.1 |
| Serine | - | - | - | X | - | X | X | - | - | 73 | UCU, UCC, UCA, UCG, AGU,AGC | 6.8 |
| Threonine | X | - | - | X | - | X | - | - | - | 93 | ACU, ACC, ACA, ACG | 5.9 |
| Valine | X | - | - | - | - | X | - | - | X | 105 | GUU, GUC, GUA, GUG | 6.6 |
| Tryptophan | X | - | - | X | - | - | - | X | - | 163 | UGG | 1.4 |
| Tyrosine | X | - | - | X | - | - | - | X | - | 141 | UAU, UAC | 3.2 |
Nonstandard amino acids
Aside from the twenty standard amino acids, there is a vast number of nonstandard amino acids not used in the body's regular manufacturing of proteins. Examples of nonstandard amino acids include the selenium-containing taurine and the neurotransmitters GABA and dopamine.
Nonstandard amino acids are usually formed through post-translational modifications to standard amino acids. For example, taurine can be formed by the decarboxylation of cysteine, while dopamine is synthesized from tyrosine.
Uses of substances derived from amino acids
- Monosodium glutamate is a food additive to enhance flavor.
- L-DOPA (L-dihydroxyphenylalanine) is a drug used to treat Parkinsonism.
- 5-HTP (5-hydroxytryptophan) has been used to treat neurological problems associated with PKU (phenylketonuria), as well as depression (as an alternative to L-Tryptophan).
References
- Doolittle, R.F. (1989) Redundancies in protein sequences. In Predictions of Protein Structure and the Principles of Protein Conformation (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623
- David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry, 3rd edition, 2000, Worth Publishers, ISBN 1572591536
- On the hydrophobic nature of cysteine. (http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-3XB0N6H-H&_coverDate=09%2F10%2F1999&_alid=241945989&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=4938&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=3cb10a335716303532fc517906a12b3a)ca:Aminoàcid
cs:Aminokyselina da:Aminosyre de:Aminosäuren es:Aminoácido eo:Aminoacido fa:اسیدهای آمینه fr:Acide aminé ko:아미노산 it:Amminoacidi he:חומצת אמינו lt:Amino rūgštis hu:Aminosav nl:Aminozuur ja:アミノ酸 pl:Aminokwas pt:Aminoácido ru:Аминокислоты sl:aminokislina su:Asam amino fi:Aminohappo sv:Aminosyra zh:氨基酸