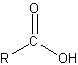
Carboxylic acid
|
|
Carboxylic acids, also known as alkanoic acids, are organic acids characterized by the presence of a carboxyl group and have the general chemical formula R-C(=O)-OH, also written as R-COOH, where R is a hydrogen or an alkyl group. The salts and anions of carboxylic acids are called carboxylates.
| Contents |
Acidity, electron distribution and resonance
Carboxylic acids are typically weak acids that partially dissociate into H+ cations and RCOO- anions in aqueous solution. The carboxylate anion R-COO- is usually named with the suffix "-ate", so acetic acid, for example, becomes acetate ion. Only about 0.02% of all acetic acid molecules are dissociated at room temperature in solution.
The two electronegative oxygen atoms tend to pull the electron away from the hydrogen of the hydroxyl group, and the remaining proton H+ can more easily leave. The remaining negative charge is then distributed symmetrically among the two oxygen atoms, and the two carbon–oxygen bonds take on a partial double bond character (i.e., they are delocalised).
This is a result of the resonance structure created by the carbonyl component of the carboxylic acid, without which the OH group does not as easily lose its H+ (see alcohol).
The presence of electronegative groups (such as -OH or -Cl) next to the carboxylic group increases the acidity. So for example, trichloroacetic acid (three -Cl groups) is a stronger acid than lactic acid (one -OH group) which in turn is stronger than acetic acid (no helping group).
Reactions
Carboxylic acids can be made by the complete oxidation of primary alcohols. This can be done with sodium dichromate, Sulphuric acid and heat.
Carboxylic acids react with bases to form carboxylate salts, in which the hydrogen of the -OH group is replaced with a metal ion. Thus, ethanoic acid (the same as acetic acid) reacts with sodium bicarbonate (baking soda) to form sodium ethanoate (sodium acetate), carbon dioxide, and water:
- CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O
Carboxyl groups also react with amine groups to form peptide bonds and with alcohols to form esters.
Carboxylic acids react with Sulfonyl dichloride (SO2Cl2) to form acyl chlorides these are extremely reactive and useful to synthesise other organic compounds.
Carboxylic acids can be reduced by LiAlH4 to form primary alcohols:
- Missing image
COOH_reduced_by_LAH.png
Image:COOH_reduced_by_LAH.png
Examples
Some carboxylic acids include:
- HCOOH formic acid (found in insect stings, formic refers to ants)
- all fatty acids, where R is an alkane in saturated acids and an alkene in unsaturated acids
- CH3COOH acetic or ethanoic acid (found in vinegar)
- CH3CH2COOH propanoic acid
- C6H5COOH benzoic acid (sodium benzoate, the sodium salt of benzoic acid is used as a food preservative)
- butyric acid
- CH2=CHCOOH acrylic acid
- lactic acid, found in sour milk
- oxalic acid
- malonic acid
- succinic acid
- glutaric acid
- adipic acid
- salicylic acid
- all amino acids
See also
de:Carbonsäure et:Karboksüülhape es:Grupo carboxilo fr:Acide carboxylique lv:Karbonskābes nl:Carbonzuur nn:Karboksylsyre ja:カルボン酸 pl:Kwas karboksylowy su:Asam karboksilat fi:Karboksyylihappo