Alkene
|
|
| |
| Properties | |
|---|---|
| General formula | CnH2n |
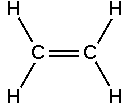
An alkene is an unsaturated hydrocarbon containing at least one carbon-carbon double bond. Alkenes form a homologous series, the alkenes with general formula CnH2n. The alkenes is one of three homologous series that contain carbon-carbon double bonds; the other two being the alkynes and the arenes.
The simplest alkene is C2H4, which has the common name "ethylene" and the IUPAC name "ethene".
| Contents |
Structure of Alkenes
Shape of Alkenes
As predicted by the VSEPR model of electron pair replusion (see covalent bond), the bond angles about each carbon in a double bond are about 120°, although the angle may be larger because of strain introduced by nonbonded interactions created by groups attached to the carbons of the double bond. For example, the C-C-C bond angle in propene (propylene) is 123.9°.
See also: molecular geometry
Molecular Geometry Carbon-Carbon Double Bond
Like single covalent bonds, double bonds can be described in terms of overlapping atomic orbitals, except that unlike a single bond (which consist of a single sigma bond), a carbon-carbon double bond consists of one sigma bond and one pi bond.
Each carbon of the double bond uses its three sp2 hybrid orbitals to form sigma bonds to three atoms. The unhybridized 2p atomic orbitals, which lie perpendicular to the plane created by the axes of the three sp2 hybrid orbitals, combine to form the pi bond.
Because it requires a large amount of energy to break a pi bond (264 kJ/mol in ethylene), rotation about the carbon-carbon double bond is very difficult and therefore severely restricted.
See also: molecular geometry
Physical properties
- The same as alkanes.
- Physical state depends on molecular mass.
Chemical properties
Alkenes are relatively stable compounds, but are more reactive than alkanes.
Reactions
Synthesis
- The most common industrial synthesis path for alkenes is cracking of petroleum.
- Alkenes can be synthesized from alcohols via an elimination reaction that removes one water molecule:
H3C-CH2-OH + H2SO4 → H3C-CH2-O-SO3H + H2O → H2C=CH2 + H2SO4 - Catalytic synthesis of higher α-alkenes can be achieved by a reaction of ethene with triethylaluminium, an organometallic compound in the presence of nickel, cobalt or platinum.
Addition reactions
Catalytic addition of hydrogen
Catalytic hydrogenation of alkenes produce the corresponding alkanes. The reaction is carried out under pressure in the presence of a metallic catalyst. Common industrial catalysts are based on platinum, nickel or palladium, for laboratory syntheses, Raney's nickel is often employed. This is an alloy of nickel and aluminium.
This is the catalytic hydrogenation of ethylene to yield ethane:
CH2=CH2 + H2 → CH3-CH3
Electrophilic addition
Most addition reactions to alkenes follow the mechanism of electrophilic addition.
- Halogenation: Addition of elementary bromine or chlorine to alkenes yield vicinal Dibromo- and dichloroalkenes, respectively. The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes:
CH2=CH2 + Br2 → BrCH2-CH2Br - Hydrohalogenation: Addition of hydrohalic acids like HCl or HBr to alkenes yield the corresponding haloalkanes.
CH3-CH=CH2 + HBr → CH3-CHBr-CH3
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with less hydrogen substituents (Markovnikov's rule). - Addition of a carbene or carbenoid yields the corresponding cyclopropane
Oxidation
- In the presence of oxygen, alkenes burn with a bright flame to carbon dioxide and water.
- Catalytic oxidation with oxygen or the reaction with percarboxylic acids yields epoxides
- Reaction with ozone leads to the breaking of the double bond, yielding two aldehydes or ketones
R1-CH=CH-R2 + O3 → R1-CHO + R2-CHO + H2O
This reaction can be used to determine the position of a double bond in an unknown alkene.
Polymerisation
Polymerization of alkenes is an economically important reaction which yields polymers of high industrial value, such as the plastics polyethylene and polypropylene. Polymerization can either proceed via a free-radical or an ionic mechanism. For detail regarding the reaction mechanisms, see the polymerization article.
Nomenclature of Alkenes
IUPAC Names
To form the root of the IUPAC names for alkenes, simply change the -an- infix of the parent to -en-. For example, CH3-CH3 is the alkane ethANe. The name of CH2=CH2 is therefore ethENe.
In higher alkenes, where isomers exist that differ in location of the double bond, the following numbering system is used:
- Number the longest carbon chain the contains the double bond in the direction that gives the carbon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first carbon
- Name branched or substituted alkenes in a manner similar to alkanes.
- Number the carbon atoms, locate and name substituent groups, locate the double bond, and name the main chain
|
CH3CH2CH2CH2CH==CH2 6 5 4 3 2 1 1-Hexene
|
CH3 | CH3CH2CH2CH2CH==CH2 6 5 4 3 2 1 4-Methyl-1-hexene
|
CH3 | CH3CH2CH2CH2CH==CH2 6 5 4 3 |2 1 CH2CH3 2-Ethyl-4-methyl-1-hexene
|
Common Names
Despite the precision and universal acceptance of the IUPAC naming system, some alkenes are known almost exclusively by their common names:
| CH2="CH2" | CH3CH="CH2" | CH3C(CH3)="CH2" | |
| IUPAC name: | Ethene | Propene | 2-Methylpropene |
| Common name: | Ethylene | Propylene | Isobutylene |
See also:
da:Alken de:Alkene es:Alqueno fr:Alcène nl:Alkeen ja:アルケン it:Alcheni pl:Alken ru:Алкены fi:Alkeeni su:alkéna sv:Alken zh:烯烃
