Aldehyde
|
|
An aldehyde is either a functional group consisting of a terminal carbonyl group, or a compound containing a terminal carbonyl group.
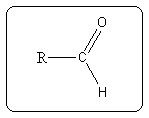 (Where -R represents the carbon chain.)
(Where -R represents the carbon chain.)
| Contents |
Structure
The aldehyde functional group is a carbonyl group bonded to a hydrogen atom and a carbon atom.
α carbon & α hydrogen
An α (alpha) carbon is a carbon adjacent to a carbonyl group. An α hydrogen is a hydrogen atom bonded to the α carbon.
The pKa of an α hydrogen is 20.
Carbonyl group
The other molecules containing a carbonyl group are:
Nomenclature
Aldehydes are named by IUPAC nomenclature by changing the suffix -e of the parent alkane to -al.
Aliphatic aldehydes are named as derivatives of their longest alkyl chain. Thus, HCHO is named as a derivative of methane, and CH3CH2CH2CHO is named as a derivative of butane. The suffix -al replaces the -e of the alkane name. Thus, HCHO is named methanal, more commonly known as formaldehyde, and CH3CH2CH2CHO is named butanal.
When a -CHO group is attached to a ring, the suffix -carbaldehyde is used. Thus, C6H11-CHO is known as cyclohexanecarbaldehyde. The name benzaldehyde is used as the root for aldehydes derived from benzene.
Physical properties
The carbonyl group is polar.
Chemistry
Preparation
There are three notable methods for preparing aldehydes:
- Reacting a primary alcohol with an oxidizing agent,
- Reacting an alkene (if there is a vinylic hydrogen) with ozone will cause the C=C bond to break yielding an aldehyde upon workup, in a process called ozonolysis.
- Reacting an ester with DIBAL-H can cause reduction, yielding an aldehyde.
The primary means of synthesis is the oxidation of a primary alcohol. In the laboratory this may be achieved by heating the alcohol with a chromium(VI) reagent an acidified solution of potassium dichromate, which is reduced to green Cr3+ during the reaction. Excess dichromate will further oxidise the aldehyde to form a carboxylic acid, so either the aldehyde is distilled out as it forms (if volatile), or milder methods such as PCC oxidation or Swern oxidation are used. The equation is shown below with propan-1-ol being oxidised to form propanal.
CH3CH2CH2OH —→ CH3CH2CHO
A similar process reacting pentan-1-ol to form pentanal is illustrated below.
Common reactions
- aldehyde + alcohol + acid or base —→ hemiacetal
- hemiacetal + alcohol + acid catalyst —→ acetal + water
- Simple hemiacetals are usually unstable, although cyclic ones such as glucose do exist. Acetals are stable, but these revert to the aldehyde in the presence of aqueous acid.
- Treating aldehydes with oxidizing agents such as potassium permanganate, nitric acid, or chromium(VI) oxide, will yield a carboxylic acid.
- Treating aldehydes with Tollens' reagent (which is prepared by adding a drop of sodium hydroxide solution into silver nitrate solution to give a precipitate of silver(I) oxide, and then adding just enough dilute ammonia solution to redissolve the precipitate in aqueous ammonia to produce [Ag(NH3)2]+ complex) will convert aldehydes to carboxylic acids without attacking carbon-carbon double bonds. See also oxidation of aldehyde (http://www.wiu.edu/users/mftkv/Chemistry102/oxidationaldehydes.html)
- Aldehydes can react with water (under acidic or basic conditions) to form hydrates, R-C(H)(OH)(OH), although these are only stable when strong electron withdrawing groups are present as in chloral hydrate. The mechanism is identical to hemiacetal formation.
- Aldehydes can react with HCN to form cyanohydrins, R-C(H)(OH)(CN).
- Treating an aldehyde with a Grignard reagent can yield an alcohol with a substituted group from the Grignard reagent.
- Treating aldehydes with hydrazine will reduce a C=O bond to CH2 via the Wolff-Kishner reaction.
Nucleophilic addition
- aldehyde + nucleophile —→ tetrahedral carbonyl addition compound
- aldehyde + ammonia or primary amine —→ tetrahedral carbonyl addition compound
- tetrahedral carbonyl addition compound + acid (catalyst) —→ imine + water
- aldehyde + ammonia or primary amine —→ tetrahedral carbonyl addition compound
Keto-enol tautomerism
Equilibration of keto and enol tautomers is catalyzed by acid.
Oxidation & Reduction
- Aldehydes are oxidized to carboxylic acids.
- Aldehydes are reduced to primary alcohols.
Examples of Aldehydes
See also
es:Aldehído eo:Aldehido fr:Aldéhyde ko:알데하이드 he:אלדהיד nl:Aldehyde ja:アルデヒド nn:Aldehyd pl:Aldehyd pt:Aldeído ru:Альдегиды su:aldehida zh:醛

