Benzene
|
|
| Benzene | |
|---|---|
| Chemical name | Benzene |
| Chemical formula | C6H6 |
| Molecular mass | 78.11 g/mol |
| Density | 0.8786 g/ml |
| Melting point | 5.5 °C |
| Boiling point | 80.1 °C |
| Heat of vaporization | 44.3 kJ/mol |
| Heat of fusion | 9.84 kJ/mol |
| CAS number | 71-43-2 |
| SMILES | C1=CC=CC=C1 |
 | |
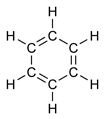
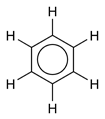
Benzene, C6H6, PhH, or benzol is a colorless and flammable liquid with a pleasant, sweet smell. Benzene is a carcinogen. It is a component of gasoline and of napalm. It is an important industrial solvent and precursor in the production of drugs, plastics, gasoline, synthetic rubber, and dyes. Benzene is a natural constituent of crude oil, but it is usually synthesized from other compounds present in petroleum. Benzene is an aromatic hydrocarbon, and the second [n]-annulene ([6]-annulene).
| Contents |
History
Benzene was discovered in 1825 by the English scientist Michael Faraday, who isolated it from oil gas and gave it the name bicarburet of hydrogen. In 1833, the German chemist Eilhard Mitscherlich produced it via the distillation of benzoic acid (from gum benzoin) and lime. Mitscherlich gave the compound the name benzin. In 1845, the English chemist Charles Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method.
Structure
The formula of benzene (C6H6), caused a mystery for some time after its discovery, as no proposed structure could take account of all the bonds (carbon usually forms four single bonds and hydrogen one).
The chemist Friedrich August Kekulé von Stradonitz was the first to deduce the ring structure of benzene. After years of studying carbon bonding, benzene and related molecules, he dreamt one night of a snake eating its own tail. (See Ouroboros.) Upon waking he was inspired to deduce the ring structure of benzene.
While his claims were well publicized and accepted, by the early 1920s Kekulé's biographer came to the conclusion that Kekulé's understanding of the tetravalent nature of carbon bonding depended on the previous research of Archibald Scott Couper (1831-1892); further, the German chemist Josef Loschmidt (1821-1895) had earlier posited a cyclic structure for benzene as early as 1862. The cyclic nature of benzene was finally confirmed by the eminent crystallographer Kathleen Lonsdale.
Benzene presents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:
Using X-ray diffraction, researchers discovered that all of the carbon-carbon bonds in benzene are of the same length, and it is known that a single bond is longer than a double bond. In addition, the bond length (the distance between the two bonded atoms) in benzene is greater than a double bond, but shorter than a single bond. There seems in effect to be a bond and a half between each carbon.
This is explained by electron delocalization. In order to picture this, we must consider the position of electrons in the bonds of benzene.
One representation is that the structure exists as a superposition of the forms below, rather than either form individually. This type of structure is called a resonance hybrid.
Missing image
Benz3.png
Benzene, mesomeric structures
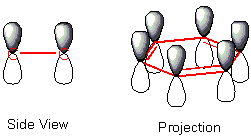
In reality, neither form really exists. Delocalisation must be explained using a higher level of theory than single and double bonds. The single bonds are formed with electrons in line between the carbon atoms - this is called σ (sigma) symmetry. Double bonds consist of a sigma bond and another, π (pi) bond. This second bond has electrons orbiting in paths above and below the plane of the ring at each bonded carbon atom. The π-bonds are formed from atomic p-orbitals above and below the plane of ring. The following diagram shows the positions of these p-orbitals:
Since they are out of the plane of the atoms, these orbitals can interact with each other freely, and become delocalised. This means that instead of being tied to one atom of carbon, each electron is shared by all six in the ring. Thus there are not enough electrons to form double bonds on all the carbon atoms, but the "extra" electrons strengthen all of the bonds on the ring equally. The resulting molecular orbital has π symmetry.
Missing image
Benzene-orbitals2.png
Benzene orbital delocalisation
This delocalisation of electrons is known as aromaticity, and gives benzene great stability. This is the fundamental property of aromatic chemicals which differentiates them from non aromatics.
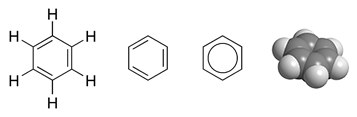
To reflect the delocalised nature of the bonding, benzene may be depicted as a circle inside a hexagon in chemical structure diagrams:
As is common in diagrams of molecular structures, the carbon atoms in the diagram above have been left unlabeled.
Benzene occurs sufficiently often as a component of organic molecules that there is a Unicode symbol with the code 232C to represent it: ⌬
Note: Many fonts do not have this Unicode character, so your browser may not be able to display it correctly.
Substituted benzenes
Many important chemicals are essentially benzene, with one or more of the hydrogen atoms replaced with another functional group:
Alkyl substituents (aklylbenzenes)
- toluene C6H5-CH3
- xylene C6H4(-CH3)2
- mesitylene C6H3(-CH3)3
Other substituents
- phenol C6H5-OH
- aniline C6H5-NH2
- chlorobenzene C6H5-Cl
- nitrobenzene C6H5-NO2
- picric acid C6H2(-OH)(-NO2)3
- trinitrotoluene C6H2(-CH3)(-NO2)3
- benzoic acid C6H5-COOH
- salicylic acid C6H4(-OH)(-COOH)
- acetylsalicylic acid C6H4(-O-C(=O)-CH3)(-COOH)
- paracetamol C6H4(-NH-C(=O)-CH3)(-OH)
- phenacetin C6H4(-NH-C(=O)-CH3)(-O-CH2-CH3)
Fused aromatic rings
- naphthalene
- anthracene
- phenanthrene
- indole
- benzofuran
- quinoline
- isoquinoline
- polycyclic aromatic hydrocarbons (PAH)
Heterocyclic analogs
In heterocycles, carbon atoms in the benzene ring are replaced with another element:
Production
Benzene may result whenever carbon-rich materials undergo incomplete combustion. It is produced naturally in volcanoes and forest fires, and is also a component of cigarette smoke.
Up until World War II, most benzene was produced as a byproduct of coke production in the steel industry. However, in the 1950s, increased demand for benzene, especially from the growing plastics industry, necessitated the production of benzene from petroleum. Today, most benzene comes from the petrochemical industry, with only a small fraction being produced from coal.
Three chemical processes contribute about equally to industrial benzene production: catalytic reforming, toluene hydrodealkylation, and steam cracking.
Catalytic reforming
In catalytic reforming, a mixture of hydrocarbons with boiling points between 60-200°C is blended with hydrogen gas, then exposed to a platinum chloride or rhenium chloride catalyst at 500-525°C and pressures ranging from 8-50 atm. Under these conditions, aliphatic hydrocarbons form rings and lose hydrogen to become aromatic hydrocarbons. The aromatic products of the reaction are then separated from the reaction mixture by extraction with any one of a number of solvents, including diethylene glycol or sulfolane, and benzene is then separated from the other aromatics by distillation.
Toluene hydrodealkylation
Toluene hydrodealkylation converts toluene to benzene. In this process, toluene is mixed with hydrogen, then passed over a chromium, molybdenum, or platinum oxide catalyst at 500-600°C and 40-60 atm pressure. Sometimes, higher temperatures are used instead of a catalyst. Under these conditions, toluene undergoes dealkylation according to the chemical equation:
Typical reaction yields exceed 95%. Sometimes, xylene and heavier aromatics are used in place of toluene, with similar efficiency.
Steam cracking
Steam cracking is the process used to produce ethylene and other olefins from aliphatic hydrocarbons. Depending on the feedstock used to produce the olefins, steam cracking can produce a benzene-rich liquid byproduct called pyrolysis gasoline. Pyrolysis gasoline can be blended with other hydrocarbons as a gasoline additive, or distilled to separate it into its components, including benzene.
Uses
Prior to the 1920's, benzene was frequently used as an industrial solvent, especially for degreasing metal. As its toxicity became obvious, other solvents replaced benzene in applications that directly exposed the user to benzene.
As a gasoline additive, benzene increases the octane rating and reduces knocking. As a result, gasoline often contained several percent benzene before the 1950s, when tetraethyl lead replaced it as the most widely used antiknock additive. However, with the global phaseout of leaded gasoline, benzene has made a comeback as a gasoline additive in some nations. In the United States, concern over its negative health effects and the possibility of benzene entering the groundwater have led to stringent regulation of gasoline's benzene content, with values around 1% typical. European gasoline specifications now contain the same 1% limit on benzene content.
By far the largest use of benzene is an intermediate to make other chemicals. The most widely produced derivatives of benzene are styrene, which is used to make polymers and plastics, phenol for resins and adhesives (via cumene), and cyclohexane, which is used in Nylon manufacture. Smaller amounts of benzene are used to make some types of rubbers, lubricants, dyes, detergents, drugs, explosives and pesticides.
Health effects
Breathing very high levels of benzene can result in death, while high levels can cause drowsiness, dizziness, rapid heart rate, headaches, tremors, confusion, and unconsciousness. Eating or drinking foods containing high levels of benzene can cause vomiting, irritation of the stomach, dizziness, sleepiness, convulsions, rapid heart rate, and death.
The major effect of benzene from chronic (long term) exposure is to the blood. Benzene damages the bone marrow and can cause a decrease in red blood cells leading to anemia. It can also cause excessive bleeding and depress the immune system, increasing the chance of infection.
Some women who breathed high levels of benzene for many months had irregular menstrual periods and a decrease in the size of their ovaries. It is not known whether benzene exposure affects the developing fetus in pregnant women or fertility in men.
Animal studies have shown low birth weights, delayed bone formation, and bone marrow damage when pregnant animals breathed benzene.
The US Department of Health and Human Services (DHHS) classifies benzene as a human carcinogen. Long-term exposure to high levels of benzene in the air can cause leukemia, a fatal cancer of the blood-forming organs.
Several tests can show if you have been exposed to benzene. There is a test for measuring benzene in the breath; this test must be done shortly after exposure. Benzene can also be measured in the blood; however, since benzene disappears rapidly from the blood, measurements are accurate only for recent exposures.
In the body, benzene is metabolized. Certain metabolites can be measured in the urine. However, this test must be done shortly after exposure and is not a reliable indicator of how much benzene you have been exposed to, since the same metabolites may be present in urine from other sources.
The US Environmental Protection Agency has set the maximum permissible level of benzene in drinking water at 0.005 milligrams per liter (0.005 mg/L). The EPA requires that spills or accidental releases into the environment of 10 pounds (4.5 kg) or more of benzene be reported to the EPA.
The US Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 1 part of benzene per million parts of air (1 ppm) in the workplace during an 8-hour workday, 40-hour workweek.
Benzene exposure
Workers in various industries that make or use benzene may be at risk for being exposed to high levels of this carcinogenic chemical. Industries that involve the use of benzene include the rubber industry, oil refineries, chemical plants, shoe manufacturers, and gasoline related industries. In 1987, OSHA estimated that about 237,000 workers in the United States were potentially exposed to benzene, and it is not known if this number has substantially changed since then.
See also
- Simple aromatic ring for analogs of benzene
External links
- Loschmidt's Benzene structure (http://www.physicstoday.org/pt/vol-54/iss-3/captions/p45cap4.html)
- Benzene Material Safety Data Sheet (http://www.hazard.com/msds/f2/bqv/bqvjq.html)
References
- Archibald Scott Couper, On a New Chemical Theory, Philosophical Magazine 16, 104-116 (1858)
- Josef Loschmidt, Chemische Studien I, Carl Gerold's Sohn, Vienna (1861),
- Josef Loschmidt, Chemische Studien I, Aldrich Chemical Co, Milwaukee (1989), catalog no. Z-18576-0, and (1913) catalog no. Z-18577-9
- Kathleen Lonsdale, "The Structure of the Benzene Ring in Hexamethylbenzene," Proceedings of the Royal Society 123A: 494 (1929).
- Kathleen Lonsdale, "An X-Ray Analysis of the Structure of Hexachlorobenzene, Using the Fourier Method," Proceedings of the Royal Society 133A: 536 (1931).da:Benzen
de:Benzol es:Benceno eo:Benzeno fr:Benzène it:Benzene he:בנזן nl:Benzeen ja:ベンゼン pl:Benzen ru:Бензол uk:Бензол zh:苯