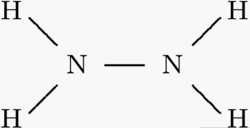Properties
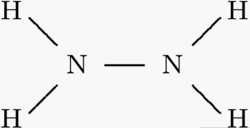
The structure formula of Hydrazine. |
|
General
|
| Name |
Hydrazine |
| Chemical formula |
N2H4 |
| Appearance |
Colourless liquid |
|
Physical
|
| Formula weight |
32.0 amu |
| Melting point |
274 K (1 °C) |
| Boiling point |
387 K (114 °C) |
| Density |
1.01g/ml |
| Solubility |
very soluble |
|
Thermochemistry
|
| ΔfH0gas |
95.35 kJ/mol |
| ΔfH0liquid |
50.63 kJ/mol |
| ΔfH0solid |
37.63 kJ/mol |
| S0gas, 1 bar |
238.66 J/mol·K |
| S0liquid, 1 bar |
121.52 J/mol·K |
| S0solid |
? J/mol·K |
|
Safety
|
| Ingestion |
Extremly Toxic, possibly carcinogenic |
| Inhalation |
Very dangerous—extremely destructive to the upper respiratory tract |
| Skin |
Can cause severe burns, can be absorbed into bloodstream |
| Eyes |
Can cause permanent damage |
| More info |
Hazardous Chemical Database (http://www.hhmi.org/research/labsafe/lcss/lcss46.html) |
| LD50
| as low as 25mg/kg
|
|
SI units were used where possible. Unless otherwise stated, standard conditions were used.
Disclaimer and references
</font>
|
</table>
Hydrazine is a chemical compound with formula
N2H4 used as a rocket fuel.
Hydrazine is a liquid with weak basic properties similar to ammonia. Due to the alpha effect the nucleophilicity is much stronger than that of ammonia, which makes it more reactive. It can be made by oxidizing ammonia with sodium hypochlorite (the Raschig process). It is a monopropellant rocket fuel.
Hydrazine derivatives 1,1-dimethylhydrazine and 1,2-dimethylhydrazine, in which two of the hydrogen atoms are substituted with methyl groups, are also described as hydrazines.
1,1-Dimethylhydrazine is used to make hypergolic (self-igniting) bipropellant rocket fuels.
Health effects
Breathing hydrazines for short periods may cause coughing and irritation of the throat and lungs, convulsions, tremors, or seizures. Breathing hydrazines for long periods may cause liver and kidney damage, as well as serious effects on reproductive organs.
Eating or drinking small amounts of hydrazines may cause nausea, vomiting, uncontrolled shaking, inflammation of the nerves, drowsiness, or coma. Found in chewing tobacco and cigarettes.
Tumors have been seen in many organs of animals that were exposed to hydrazines by ingestion or breathing, but most tumors have been found in the lungs, blood vessels, or colon. 1,2-Dimethylhydrazine has caused colon cancer in laboratory animals following a single exposure.
The Department of Health and Human Services (DHHS) has determined that hydrazine and 1,1-dimethylhydrazine are known carcinogens. The International Agency for Research on Cancer (IARC) has determined that hydrazine, 1,1-dimethylhydrazine, and 1,2-dimethylhydrazine are possible human carcinogens. The Environmental Protection Agency (EPA) has determined that hydrazine, 1,1-dimethylhydrazine, and 1,2-dimethylhydrazine are probable human carcinogens.
The American Conference of Governmental Industrial Hygienists (ACGIH) currently lists hydrazine and 1,1-dimethylhydrazine as suspected carcinogens, but has recently recommended that the listing of hydrazine be changed to that of animal carcinogen, not likely to cause cancer to people under normal exposure conditions.
The False Morel contains the chemical gyromitrin, which is metabolized into monomethyl hydrazine inside the body. Consequently, the toxic effects of this mushroom are the same as with hydrazine poisoning.
Use
Hydrazine is used primarily as a chemical intermediate to produce agricultural chemicals, spandex fibers, and antioxidants. Hydrazine is also used as rocket fuel, an oxygen scavenger in water boilers and heating systems, a polymerization catalyst, a blowing agent, and as a scavenger for gases. Additionally, it is used for plating metals on glass and plastics and in fuel cells, solder fluxes, and photographic developers. Hydrazine is used as a reactant in fuel cells in the military, as a reducing agent in electrodeless nickel plating, as a chain extender in urethane polymerizations, as a reducing agent in plutonium extraction from reactor waste, and as a water treatment chemical. Hydrazine is also used as a chemical intermediate for blowing agents, photography chemicals, pharmaceuticals, antituberculants, textile dyes, heat stabilizers, explosives, and to make hydrazine sulfate. In addition, it has recently been determined that hydrazine increases the speed of the thin-film transistors used in liquid crystal displays, a discovery that promises to revolutionize the manufacture of LCD computer monitors. Hydrazine in a 70% solution is used to power the EPU (Emergency Power Unit) on the F-16 fighter plane. Hydrazine is also used as low-power propellant for Space Shuttle maneuvers in orbit, as Hydrazine will burn in the absence of oxygen.de:Hydrazin
fr:Hydrazine
it:Idrazina
nl:Hydrazine
pl:Hydrazyna