Ester
|
|
- For other uses, see Ester (disambiguation).
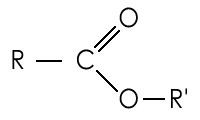
In organic chemistry and biochemistry esters are substances that have the functional group (R-COO-R') (the carbon is double-bonded to one oxygen atom and single-bonded to another) and consist of an alkane united with the residue of any oxygen acid, organic or inorganic. An ester is a product of the reaction of an acid (usually organic) and an alcohol (the hydrogen of the acid R-COOH is replaced by an alkyl group R"). Esters mainly result from the condensation (this is, a reaction that produces water) of a carboxylic acid and an alcohol. The process is called esterification. This reaction can be catalysed by the presence of H+ ions. Sulphuric acid is often used as a catalyst for this reaction. The name ester is derived from the German Essig-Aether, an old name for acetic acid ethyl ester (ethyl acetate).
This is the general displayed formula of an ester:
| Contents |
Naming of esters
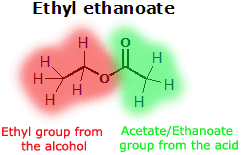
Esters can be produced by an equilibrium reaction between an alcohol and a carboxylic acid. The ester is named according to the alkyl group (the part from the alcohol) and acetate (the part from the carboxylic acid) which make it up; for example, the reaction between methanol and butanoic acid yields the ester methyl butanoate (as well as water).
The simplest ester is H-COO-CH3 (methyl formate, also called methyl methanoate). The hydrogen atom on the left can be replaced with a CH3 group or additional CH2 units, producing other methyl esters, including methyl stearate, a component of biodiesel.
Physicals
Esters can participate in hydrogen bonds as hydrogen-bond acceptors, but cannot act as hydrogen-bond donors, unlike their parent alcohols. This ability to participate in hydrogen bonding makes them more water soluble than their parent hydrocarbons. However, the limitations on their hydrogen bonding also make them more hydrophobic than either their parent alcohols or parent acids. Their lack of hydrogen bond donating ability means that ester molecules cannot hydrogen bond to each other, which makes esters generally more volatile than an carboxylic acid of similar molecular weight. This property makes them very useful in organic analytical chemistry: unknown organic acids with low volatility can often be esterified into a volatile ester which can then be analysed using gas chromatography, gas liquid chromatography, or mass spectrometry.
Many esters have distinctive odors, which has led to their widespread use as artificial flavorings and fragrances. For example:
- methyl butanoate smells of pineapple
- methyl salicylate (oil of wintergreen) smells of the ointments called Germolene™ and Ralgex™ in the UK
- methyl benzoate smells of marzipan
- ethyl methanoate smells of raspberry
- ethyl butanoate smells of pineapple
- ethyl salicylate smells of mint
- pentyl ethanoate smells of banana
- pentyl pentanoate smells of apple
- pentyl butanoate smells of pear or apricot
- octyl ethanoate smells of orange
Esters may undergo hydrolysis - the breakdown of an ester by water. This process can be catalyzed both by acids and bases. The base-catalyzed process is called saponification. The hydrolysis yields an alcohol and a carboxylic acid or its carboxylate salt.
Ester_hydrolysis.PNG
See also
External links
- Molecule of the month: Ethyl acetate and other esters (http://www.chm.bris.ac.uk/motm/ethylacetate/ethylh.htm)de:Ester
et:Estrid es:Éster eo:Estero fr:Ester he:אסטר nl:Ester (chemie) ja:エステル lv:Esteri nn:Ester pl:Ester pt:Ester sv:Ester (kemikalie)