Polar molecule
|
|
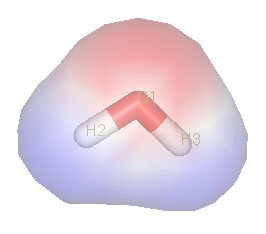
A commonly-used example of a polar compound is water (H2O). The electrons of water's hydrogen atoms are strongly attracted to the oxygen atom, and are actually closer to oyxgen's nucleus than to the hydrogen nuclei; thus, water has a relatively strong negative charge in the middle (red shade), and a positive charge at the ends (blue shade).
In chemistry, a polar molecule is a molecule in which the centers of positive and negative charge distribution do not converge. These molecules are characterized by a dipole moment which measures their polarity.
Polar compounds are highly soluble in other polar compounds, and virtually insoluble in nonpolar compounds.
See Also
See also:
