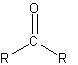
Carbonyl
|
|
In chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom.
A carbonyl group characterizes the following types of compounds (and a representation of the full group, where -CO means a C=O group):
- aldehyde- (R-CHO)
- ketone- (R-CO-R1)
- carboxylic acid- (R-COOH)
- ester- (R-CO-O-R1)
- amide- (R-CO-NH2 or R-CO-NHR1 or R-CO-NR1R2)
- enone- R2-C=CR−CO−R
- acyl chloride- (R-CO-Cl)
- anhydride- (R-CO-O-CO-R)
| Contents |
Reactivity
Oxygen is more electronegative than carbon, and thus pulls electron density away from carbon to increase the bond's polarity. Therefore, the carbonyl carbon becomes electrophilic, and thus more reactive with nucleophiles.
Carbonyl groups can be reduced by reaction with hydride reagents such as NaBH4 and LiAlH4, and by organometallic reagents such as organolithium reagents and Grignard reagents.
Other important reactions include:
- Wolff-Kishner Reduction
- Clemmensen reduction
- Conversion into thioacetals
- Hydration to hemiacetals and hemiketals, and then to acetals and ketals
- Reaction with ammonia and primary amines to form imines
- Reaction with hydroxyl amines to form oximes
- Reaction with cyanide to form cyanohydrins
Spectroscopy
- IR spectroscopy: the C=O double bond absorbs infrared light at wavenumbers between approximately 1680–1750 cm-1. This absorption is known as the "carbonyl stretch" when displayed on an infrared absorption spectrum.
- Nuclear magnetic resonance: the C=O double bond exhibits different resonances depending on surrounding atoms.
- Mass spectrometry
See Also
References
- William Reusch. (2004) Aldehydes and Ketones (http://www.cem.msu.edu/~reusch/VirtualText/aldket1.htm) Retrieved 23 May 2005.
- ILPI. (2005) The MSDS Hyperglossary- Anhydride (http://www.ilpi.com/msds/ref/anhydride.html).
Further Reading
- L.G. Wade, Jr. Organic Chemistry, 5th ed. (http://www.amazon.com/exec/obidos/tg/detail/-/013033832X/qid=1116901319/sr=8-1/ref=pd_csp_1/002-6183595-6998447?v=glance&s=books&n=507846)] Prentice Hall, 2002. ISBN 013033832X
- The Frostburg State University Chemistry Department. Organic Chemistry Help (http://www.chemhelper.com/) (2000).
- Advanced Chemistry Development, Inc. IUPAC Nomenclature of Organic Chemistry (http://www.acdlabs.com/iupac/nomenclature) (1997).
- William Reusch. VirtualText of Organic Chemistry (http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm) (2004).
de:Carbonylgruppe fr:Groupement carbonyle ja:カルボニル基 nl:Carbonylgroep pl:Grupa karbonylowa ru:Карбонильная группа