Protein
|
|
A protein (in Greek πρωτεϊνη = first element) is a complex, high molecular weight organic compound that consists of amino acids joined by peptide bonds. Proteins are essential to the structure and function of all living cells and viruses. Many proteins are enzymes or subunits of enzymes. Other proteins play structural or mechanical roles, such as those that form the struts and joints of the cytoskeleton. Still more functions filled by proteins include immune response and the storage and transport of various ligands. In nutrition, proteins serve as the source of amino acids for organisms that do not synthesize those amino acids natively.
Proteins are one of the classes of bio-macromolecules, alongside polysaccharides, lipids, and nucleic acids, that make up the primary constituents of living things. They are amongst the most actively studied molecules in biochemistry and were discovered by Jöns Jakob Berzelius, in 1838.
Most natural proteins are encoded by DNA. DNA is transcribed to yield RNA, which serves as a template for translation by ribosomes.
| Contents |
Structure
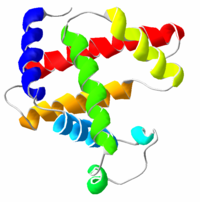
Proteins are amino acid chains that fold into unique 3-dimensional structures. The shape into which a protein naturally folds is known as its native state, which is determined by its sequence of amino acids. Biochemists refer to four distinct aspects of a protein's structure:
- Primary structure: the amino acid sequence
- Secondary structure: highly patterned sub-structures—alpha helix and beta sheet—or segments of chain that assume no stable shape. Secondary structures are locally defined, meaning that there can be many different secondary motifs present in one single protein molecule.
- Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structural motifs to one another
- Quaternary structure: the shape or structure that results from the union of more than one protein molecule, usually called subunit proteins subunits in this context, which function as part of the larger assembly or protein complex.
In addition to these levels of structure, proteins may shift between several similar structures in performing their biological function. In the context of these functional rearrangements, these tertiary or quaternary structures are usually referred to as "conformations," and transitions between them are called conformational changes.
The primary structure is held together by covalent peptide bonds, which are made during the process of translation. The secondary structures are held together by hydrogen bonds. The tertiary structure is held together primarily by hydrophobic interactions but hydrogen bonds, ionic interactions, and disulfide bonds are usually involved too.
The process by which the higher structures form is called protein folding and is a consequence of the primary structure. Although any unique polypeptide may have more than one stable folded conformation, each conformation has its own biological activity and only one conformation is considered to be the active, or native conformation.
The two ends of the amino acid chain are referred to as the carboxy terminus (C-terminus) and the amino terminus (N-terminus) based on the nature of the free group on each extremity.
Functions
Proteins are involved in practically every function performed by a cell, including regulation of cellular functions such as signal transduction and metabolism. For example, protein catabolism requires only a few enzymes termed proteases.
Mechanisms of protein regulation
Various molecules and ions are able to bind to specific sites on proteins. These sites are called binding sites. They exhibit chemical specificity. The particle that binds is called a ligand. The strength of ligand-protein binding is a property of the binding site known as affinity.
Since proteins are involved in practically every function performed by a cell, the mechanisms for controlling these functions therefore depend on controlling protein activity. Regulation can involve a protein's shape or concentration. Some forms of regulation include:
- Allosteric modulation: When the binding of a ligand at one site on a protein affects the binding of ligand at another site.
- Covalent modulation: When the covalent modification of a protein affects the binding of a ligand or some other aspect of the protein's function.
Diversity
Proteins are generally large molecules, having molecular masses of up to 3,000,000 (the muscle protein titin has a single amino acid chain 27,000 subunits long). Such long chains of amino acids are almost universally referred to as proteins, but shorter strings of amino acids are referred to as "polypeptides," "peptides" or very rarely "oligopeptides". The dividing line is somewhat undefined, although a polypeptide may be less likely to have tertiary structure and may be more likely to act as a hormone (like insulin) rather than as an enzyme or structural element.
Proteins are generally classified as soluble, filamentous or membrane-associated (see integral membrane protein). Nearly all the biological catalysts known as enzymes are proteins. (Certain RNA sequences were shown in the late 20th century to have catalytic properties as well.) Membrane-associated exchangers and ion channels, which move their substrates from place to place but do not change them; receptors, which do not modify their substrates but may simply shift shape upon binding them; and antibodies, which appear to do nothing more than bind, all are proteins as well. The filamentous material that makes up the cytoskeleton of cells and much of the structure of animals is also protein: microtubules, actin, intermediate filaments, collagen and keratin are components of skin, hair, and cartilage. Another class are the motor proteins such as myosin, kinesin, and dynein. Muscles are composed largely of the proteins myosin and actin.
Protein_Composite.jpg
Working with proteins
Proteins are sensitive to their environment. They may only be active in their native state, over a small pH range, and under solution conditions with a minimum quantity of electrolytes. A protein in its native state is often described as folded. A protein that is not in its native state is said to be denatured. Denatured proteins generally have no well-defined secondary structure. Many proteins denature and will not remain in solution in distilled water.
One of the more striking discoveries of the 20th century was that the native and denatured states in many proteins were interconvertible, that by careful control of solution conditions (by for example, dialyzing away a denaturing chemical), a denatured protein could be converted to native form. The issue of how proteins arrive at their native state is an important area of biochemical study, called the study of protein folding.
Through genetic engineering, researchers can alter the sequence and hence the structure, "targeting", susceptibility to regulation and other properties of a protein. The genetic sequences of different proteins may be spliced together to create "chimeric" proteins that possess properties of both. This form of tinkering represents one of the chief tools of cell and molecular biologists to change and to probe the workings of cells. Another area of protein research attempts to engineer proteins with entirely new properties or functions, a field known as protein engineering.
Protein and nutrition
In carnivores protein is one of the largest component of the diet. The metabolism of proteins by the body releases ammonia, an extremely toxic substance. It is then converted in the liver into urea, a much less toxic chemical, which is excreted in urine. Some animals convert it into uric acid instead.
Protein nutrition in humans
In terms of human nutritional needs, proteins differ in their ability to provide all eight of the amino acids (threonine, valine, tryptophan, isoleucine, leucine, lysine, phenylalanine, and methionine) that humans cannot produce themselves. While all natural foods contain all of these amino acids, some contain less of one or more of them (called limiting amino acids), which means that if this kind of protein was the only kind eaten over one or several days the body may not fully utilize all of it. Formerly, this effect was expressed in the form of the so-called biological value (BV), which has since found to be flawed and has been superseded by the PDCAAS. This measure may still be considered incomplete, since human diets, except in times of famine, almost never contain only one kind of protein - however, calculating the PDCAAS of a diet solely based on the PDCAAS of the individual constituents is impossible. This is because one food may provide an abundance of an amino acid that the other is missing, which means that in this case the PDCAAS of the diet is higher than that of any one of the constituents. To arrive at the final result, all individual amino acids would have to be taken into account, though, so the PDCAAS of each constituent is largely useless.
For example, grain protein has a PDCAAS of about 0.4 to 0.5, limited by lysine. On the other hand, it contains more than enough methionine. White bean protein (and that of many other pulses) has a PDCAAS of 0.6 to 0.7, limited by methionine, and contains more than enough lysine. When both are eaten in roughly equal quantities in a diet, the PDCAAS of the combined constituent is 1.0, because each constituent's protein is complemented by the other.
A more extreme example would be the combination of gelatine, which contains virtually no tryptophan and thus has a PDCAAS of 0, with isolated tryptophan, which, lacking all other essential amino acids, also has a PDCAAS of 0. The combination of both in adequate amounts has a positive PDCAAS, though, with the limiting amino acids isoleucine, threonine and methionine.
It has been claimed that, since vegans do not consume "complete" (PDCAAS 1.0) animal-derived proteins such as egg, milk, meat and fish, they must perform this kind of protein combining on a daily base to avoid suffering from protein deficiency. This idea was popularized by Frances Moore Lappé, author of Diet for a Small Planet, who subsequently renounced the idea. This view is widely criticized in vegetarian circles. The critics claim that:
- When a variety of plant foods is consumed, the PDCAAS of the total diet approaches 1, even if no conscious protein combining is performed.
- Research has shown that the body maintains amino acid pools that only need to be repleted once every few days. This means that the relevant PDCAAS is not that of any single meal, but that of two or three day's worth of food. Considering the law of large numbers, this PDCAAS will be much closer to 1, if a variety of plant proteins is consumed.
- Human protein requirements are much lower than once assumed. A suboptimal PDCAAS is thus easily overcome if more than minimum protein is consumed. Peanuts, soy and other legumes, the alga spirulina and certain grains are some of the richest sources of plant protein.
- Popular meat and milk-replacement products contain soy, which is a "complete" protein as well. Soy is also very rich in protein. Soy milk without added sugar contains almost twice as much protein per calorie as cow's milk, and about five times as much as human milk.
Human bodies can make use of all amino acids normally obtained from food for synthesizing new proteins, but the inessential ones need not be supplied by the diet, since they can be synthesized from other amino acids.
Protein deficiency can lead to symptoms such as fatigue, insulin resistance, hair loss, loss of hair pigment (hair that should be black becomes reddish), loss of muscle mass (proteins repair muscle tissue), low body temperature, and hormonal irregularities. Severe protein deficiency, encountered only in times of famine, is fatal.
Excess protein can cause problems as well, such as causing the immune system to overreact, liver dysfunction from increased toxic residues, bone loss due to increased acidity in the blood, foundering (foot problems) in horses, and has also been linked to obesity.
Proteins can often figure in allergies and allergic reactions to certain foods. This is because the structure of each form of protein is slightly different, and some may trigger a response from the immune system while others are perfectly safe. Many people are allergic to casein, the protein in milk; gluten, the protein in wheat and other grains; the particular proteins found in peanuts; or those in shellfish or other seafoods. It is extremely unusual for the same person to adversely react to more than two different types of proteins.
History
The first mention of the word protein, which means of first rank, were from a letter sent by Jöns Jakob Berzelius to Gerhardus Johannes Mulder on 10. July 1838, where he wrote:
- «Le nom protéine que je vous propose pour l’oxyde organique de la fibrine et de l’albumine, je voulais le dériver de πρωτειοξ, parce qu’il paraît être la substance primitive ou principale de la nutrition animale.» translated as:
Investigation of proteins and their properties had been going on since about 1800 when scientists were finding the first signs of this, at the time, unknown class of organic compounds.
See also
- Biochemistry
- Crystallography
- Denatured protein
- Intein
- Peptide
- Prion
- Proteinoid
- Protein structure prediction
- Protein targeting
- Proteome
- Proteomics
- Ribosome
- Standard curve
- Structural genomics
External links
- Proteins: Biogenesis to Degradation - The Virtual Library of Biochemistry and Cell Biology (http://www.biochemweb.org/proteins.shtml)
- Protein Research: Western Blot Protocols, Troubleshooting and Theory (http://www.westernblotting.org)
- The Protein Databank: The single worldwide repository for the processing and distribution of 3-D biological macromolecular structure data. (http://www.rcsb.org)
- Amino acid metabolism (http://web.indstate.edu/thcme/mwking/amino-acid-metabolism.html)ca:Proteïna
cs:Protein da:Protein de:Protein es:Proteína eo:Proteino fr:Protéine ko:단백질 io:Proteino it:Proteine he:חלבון lt:Baltymas zh-min-nan:Nn̄g-pe̍h-chit nl:Eiwit ja:蛋白質 pl:Białko pt:Proteína ru:Белок simple:Protein sl:Beljakovina su:Protéin fi:Proteiini sv:Protein th:โปรตีน tr:Protein zh:蛋白质
