Urea
|
|
| Urea | |
|---|---|
| |
| General | |
| Systematic name | Carbamide |
| Other names | ? |
| Molecular formula | (NH2)2CO |
| SMILES | ? |
| Molar mass | 60 g/mol |
| Appearance | white odourless solid |
| CAS number | [57-13-6] |
| Properties | |
| Density and phase | 750 kg/m3 |
| Solubility in water | 108 g/100 ml (20 °C) |
| Melting point | 133 °C (406 K) decomposes |
| Boiling point | n.a. |
| Acidity (pKa) | ? |
| Basicity (pKb) | ? |
| Chiral rotation [α]D | Not chiral |
| Viscosity | ? cP at ? °C |
| Structure | |
| Molecular shape | ? |
| Coordination geometry | trigonal planar |
| Crystal structure | ? |
| Dipole moment | ? D |
| Hazards | |
| MSDS | J.T. Baker (http://www.jtbaker.com/msds/englishhtml/U4725.htm) |
| Main hazards | ? |
| Flash point | ? °C |
| R/S statement | R: ? S: ? |
| RTECS number | ? |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | ? |
| Other cations | ? |
| Related ? | ? |
| Related compounds | ? |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
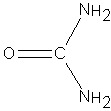
Urea is an organic compound of carbon, nitrogen, oxygen and hydrogen, with the formula CON2H4 or (NH2)2CO and the structure shown right:
Urea is also known as carbamide, especially in the recommended International Non-proprietry Names (rINN)in use in Europe e.g. the medicinal coumpound hydroxyurea (old British Approved Name) is now hydroxycarbamide.
In some animals, the individual atoms of urea come from carbon dioxide, water, aspartate and ammonia in a metabolic pathway known as the urea cycle, an anabolic process. This expenditure of energy is necessary because ammonia, a common metabolic waste product, is toxic and must be neutralized. Urea production occurs in the liver and is under the regulatory control of N-acetylglutamate. Aquatic animals do not produce urea; living in an abundant supply of water, they can simply excrete ammonia immediately as it is produced. Birds, with more severe restrictions on water consumption than most other animals, produce uric acid, a compound even less toxic than urea. Humans produce a little uric acid as a result of purine breakdown. Indeed, excess uric acid production can lead to a type of arthritis known as gout.
| Contents |
Discovery
Urea was discovered by Hilaire Rouelle in 1773. It was the first organic compound to be artificially synthesized from inorganic starting materials, in 1828 by Friedrich Woehler, who prepared it by the reaction of potassium cyanate with ammonium sulfate. This disproved the theory that the chemicals of living organisms are fundamentally different from inanimate matter and started the discipline of organic chemistry.
Industrial use
Urea's commercial uses include:
- As a raw material for the manufacture of plastics specifically, urea-formaldehyde resin.
- As a component of fertilizer and animal feed, providing a relatively cheap source of fixed nitrogen to promote growth.
- As an alternative to rock salt in the deicing of roadways and runways. It does not promote metal corrosion to the extent that salt does.
- As an ingredient in some hair conditioners and lotions.
- It is also used as a reactant in some ready-to-use cold compresses for first-aid use, due to the endothermic reaction it creates when mixed with water.
Laboratory use
Urea is a powerful protein denaturant. This property can be exploited to increase the solubility of some proteins. For this application it is used in concentrations up to 10M.
Medical Use
Drug use
Urea is used in topical dermatological products to promote rehydration of the skin.
Physiological diagnosis
See also blood urea nitrogen for a commonly performed urea test
Because urea is produced and excreted at a roughly constant rate, high levels of urea in the blood indicate a problem with the removal, or more rarely with the over-production, of urea in the body.
The most common cause of uremia is renal problems. It is measured along with creatinine to indicate direct problems with the kidneys (e.g. chronic renal failure) or secondary problems such as hypothyroidism.
Urea levels can also be increased in some malignant blood disorders, (e.g. leukaemia and multiple myeloma).
Markedly high levels of urea (uremia) can cause neurological disturbances (encephalopathy). Prolonged periods of uremia may result in the skin taking on a grey discolouration.
Other diagnostic use
Isotopically labelled urea (carbon 14 - radioactive, or carbon 13 - stable isotope) is used in the Urea breath test, which is used to detect the presence of Helicobacter pylori (H. pylori, a bacterium) in the stomach and duodenum of humans. The test detects the characteristic enzyme urease, produced by H. pylori, by a reaction which produces ammonia from urea. This reduces the pH of the stomach environment around the bacteria.
Similar bacteria species to H. pylori can be identified by the same test in animals (apes, dogs, cats-including big cats).
External link
- MSDS sheet on urea (http://www.jtbaker.com/msds/englishhtml/U4725.htm)da:Urinstof
de:Harnstoff es:Urea fr:Urée he:שתנן ja:尿素 nl:Ureum pl:Mocznik ru:Мочевина sv:Urea zh:尿素
