Secondary structure
|
|
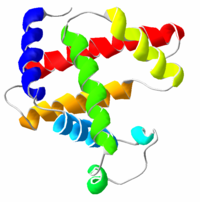
Secondary structure generally refers to how individual molecules in a biopolymer are connected to each other, e.g. whether or not individual nucleotides in an RNA molecule are connected. It does not, however, refer to their actual position in three-dimensional space; the actual positions are considered to be tertiary structure. In biochemistry and structural biology, the secondary structure of a protein includes alpha helices, beta sheets, turns, and random coil, among other less common structures. Such structures can often be detected by circular dichroism spectroscopy. Nucleic acids also have secondary structure, most notably single-stranded RNA molecules.
| Contents |
Proteins
The DSSP Code
The DSSP code is frequently used to describe the protein secondary structures with a single letter code. DSSP is an acronym for "Dictionary of Protein Secondary Structure", which was the title of the original article actually listing the secondary structure of the proteins with known 3D structure (Kabsch and Sander 1983). The secondary structure is assigned based on hydrogen bonding patterns as those initially proposed by Pauling et al. in 1951 (before any protein structure had ever been experimentally determined).
- G = 3-turn helix (3_10 helix). Min length 3 residues.
- H = 4-turn helix (alpha helix). Min length 4 residues.
- I = 5-turn helix (pi helix). Min length 5 residues.
- T = hydrogen bonded turn (3, 4 or 5 turn)
- E = beta sheet in parallel and/or anti-parallel sheet conformation (extended strand). Min length 2 residues.
- B = residue in isolated beta-bridge (single pair beta-sheet hydrogen bond formation)
- S = bend (the only non-hydrogen-bond based assignment)
In DSSP residues which are not in any of the above conformations is designated as ' ' (space), which sometimes gets designated with C (coil) or L (loop). The helices (G,H and I) and sheet conformations are all required to have a reasonable length. This means that 2 adjacent residues in the primary structure must form the same hydrogen bonding pattern. If the helix or sheet hydrogen bonding pattern is too short they are designated as T or B, respectively. Other protein secondary structure assignment categories exist (sharp turns, Omega loops etc.), but they are less frequently used.
RNA
RNA secondary structure is generally divided into helices (contiguous base pairs), and various kinds of loops (unpaired nucleotides surrounded by helices). Another reasonable definition of secondary structure of RNA is that it defines which nucleotides bind each other, and, for example, nucleotide pairs that are bound form helices. RNA secondary structure can also include pseudoknots and base triples.
For many RNA molecules, the secondary structure is highly important to the correct function of the RNA — often more so than the actual sequence. This fact aids in the analysis of non-coding RNA sometimes termed "RNA genes". RNA secondary structure can be predicted with some accuracy by computer, and many bioinformatics applications use some notion of secondary structure in analysis of RNA.
- See also : primary structure — tertiary structure — quaternary structure — translation — structural motif
Alignment
Both protein and RNA secondary structures can be used to analyze sequences by alignment. These alignments can be made more accurate by the inclusion of secondary structure information, in addition to the usual use of sequence.
Distant relationships between proteins whose primary structures are unalignable can sometimes be found by secondary structure.
Prediction
Algorithms to predict RNA secondary structure typically use dynamic programming, and many algorithms are based on Stochastic context-free grammars.
References
- W. Kabsch and C. Sander. Dictionary of Protein Secondary Structure: Pattern Recognition of Hydrogen Bonded and Geometrical Features. Biopolymers 22: 2577-2637 (1983).
- M. Zuker "Computer prediction of RNA structure", Methods in Enzymology, 180:262-88 (1989). (The classic paper on dynamic programming algorithms to predict RNA secondary structure.)
- L. Pauling and R.B Corey. Configurations of polypeptide chains with favored orientations of the polypeptide around single bonds: Two pleated sheets. Proc. Natl. Acad. Sci. USA, 37:729-740 (1951). (The original beta-sheet conformation article)
- L. Pauling, R.B. Corey and H.R. Branson. Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA, 37:205-211 (1951). (alpha- and pi-helix conformations, since they predicted that 3_10-helices would not be possible)de:Sekundärstruktur
