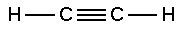Alkyne
|
|
Alkynes are hydrocarbons that have at least one triple bond between two carbon atoms. The alkynes are traditionally known as acetylenes, though the name acetylene is also used to refer specifically to the simplest member of the series, known officially as ethyne.
| Contents |
Structure
The carbon atoms in an alkyne bond are sp hybridized and carry 2 p orbitals and one sp hybrid orbital.The p orbitals overlap and create two pi bonds. In addition, two sp orbitals overlap to form one sp-sp sigma bond and now the total number of bonds is three. This makes that in the parent compound acetylene the H-C-C bond angles are 180°. because a total of 6 electrons take part in bonding this triple bond is very strong with a bond strength of 837 KJ/mole. The sigma bond contributes 369 KJ/mole, the first pi bond contributes 268 KJ/mole and the second pi bond is weak with 202 KJ/mole bond strenght. The CC bond distance with 1.21 Angstrom is also much less than that of the alkene bond which is 1.34 Angstrom or the alkane bond with 1.53 Angstrom.
Physical properties
Unlike alkanes, alkynes are unstable and very reactive. This gives rise to the intense heat of the acetylene flame used in welding.
Examples
The simplest alkyne is ethyne (acetylene):
Terminal alkynes
Terminal alkynes have one hydrogen atom as an alpha substituent.
Metal acetylides
A terminal alkyne with a strong base such as sodium,sodium amide, n-butyllithium or a grignard reagent gives the anion of the terminal alkyne and a metal acetylide. Acetylenes are fairly acidic and have pKa values (25) between that of ammonia (35) or ethanol with 16. The explanation for this acidity is that the negative charge in acetylide is stabilized as a result of the high s character of the sp orbital in which the electron pair resides. Electrons in a s orbital benefit from closer proximity to the positively charged atom nucleus and therefore lower in energy.
Synthesis
Alkynes are generally prepared by dehydrohalogenation of vicinal alkyl dihalides or the reaction of metal acetylides with primary alkyl halides. In the Fritsch-Buttenberg-Wiechell rearrangement an alkyne is prepared starting from a vinyl bromide.
Reactions
Alkynes are involved in many organic reactions.
- electrophilic addition reactions
- addition of hydrogen to the alkene or the alkane
- addition of halogens to the vinyl halides or alkyl halides
- addition of hydrogen halides to the corresponding vinyl halides or alkyl halides
- addition of water to the carbonyl compound (through the enol intermediate)
- Cycloadditions
- Diels-Alder reaction with 2-pyrone to an aromatic compound after elimination of carbon dioxide
- Huisgen 1,3-dipolar cycloaddition to Triazoles
- Bergman cyclization of enediynes to an aromatic compound
- Alkyne trimerisation to aromatic compounds
- scrambling of alkynes in alkyne metathesis to new alkyne compounds
- nucleophilic substitution reactions of metal acetylides
- new carbon-carbon bond formation with alkyl halides
- nucleophilic addition reactions of metal acetylides
- reaction with carbonyl compounds to an intermediate alkoxide and then to the hydroxyalkyne after acidic workup.
- hydroboration reaction of alkynes with organoboranes to vinylic boranes
- followed by reduction by hydrogen peroxide to the corresponding aldehyde or ketone
- oxidative cleavage with potassium permanganate to the carboxylic acidsde:Alkine
eo:Alkino fr:Alcyne he:אלקין it:Alchini nl:Alkyn ja:アルキン pl:Alkin su:alkuna sv:Alkyn zh:炔