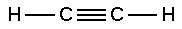Acetylene
|
|
The chemical compound acetylene, also called ethyne, was discovered in 1836 by Edmund Davy, in England; its chemical formula is C2H2 and its structure is:
Acetylene is a colorless and extremely flammable gas at standard temperature and pressure, with a melting point of -80.8°C. It normally has a pleasantly sweet ethereal odor; however, when prepared using calcium carbide, it has a sharp, noxious garlic-like odor due to impurities in the carbide forming various other compounds such as phosphine, arsine and small amounts of hydrogen sulfide and possibly very small amounts of the following potentially harmful gasses: divinyl sulfide, ammonia, methane, carbon monoxide, vinyl acetylene, divinyl acetylene, diacetylene, propadiene, hexadiene, butadienyl acetylene, and methyl acetylene. The Compressed Gas Association Commodity Specification for Acetylene has established a grading system for identifying and quantifying phosphine, arsine, and hydrogen sulfide content in commercial grades of acetylene in order to limit exposure to these impurities[1] (http://www.cganet.com/publication_detail.asp?id=G-1.1). Acetylene can explode with extreme violence if the pressure of the gas exceeds about 100 kPa as a gas or when in liquid or solid form, so it is shipped and stored dissolved in acetone. The majority of acetylene's chemical energy is contained in the carbon-carbon triple bond.
Above 400°C (which is quite low for a hydrocarbon), the pyrolysis of acetylene will start. The main products are the dimer vinylacetylene (C4H4) and benzene. At temperatures above 900°C, the main product will be soot.
The principal raw materials for acetylene manufacture are calcium carbonate (limestone) and coal. The calcium carbonate is first converted into calcium oxide and the coal into coke, then the two are reacted with each other to form calcium carbide and carbon monoxide:
- CaO + 3C → CaC2 + CO
Calcium carbide (or calcium acetylide) and water are then reacted by any of several methods to produce acetylene and calcium hydroxide.
- CaC2 + 2H2O → Ca(OH)2 + C2H2
Acetylene can also be manufactured by the partial combustion of methane with oxygen, or by the cracking of hydrocarbons.
Uses
Approximately 80 percent of the acetylene produced annually in the United States is used in chemical synthesis. The remaining 20 percent is used primarily for oxyacetylene gas welding and cutting. Combustion with oxygen produces a flame of over 3300ºC, releasing 11,800 J/g.
Acetylene is also used in the acetylene lamp or carbide lamp, formerly found in mines (not to be confused with the Davy lamp), and on vintage cars; it is still sometimes used by cavers. In this context, the acetylene is generated by adding calcium carbide (CaC2) pellets to water.
In former times a few towns used acetylene for lighting, including North Petherton in England, where it was installed in 1898.
Nowadays acetylene is used for carburization (that is, hardening) of steel. Research in the last ten years has concluded that acetylene is the best hydrocarbon available for this purpose.
External link
de:Ethin es:Acetileno fr:Acétylène it:Acetilene nl:Acetyleen ja:アセチレン pl:Etyn sv:Etyn zh:乙炔