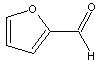
Furfural
|
|
|
General |
||
|---|---|---|
| Name | Furfural | |
| Chemical formula | C5H4O2 | |
| Formula weight | 96.09 g/mol | |
| Synonyms | 2-furancarboxaldehyde, fural, furfuraldehyde, pyromucic aldehyde | |
| CAS number | 98-01-1 | |
|
Phase behavior |
||
| Melting point | 236.2 K (-36.5 °C) | |
| Boiling point | 434.9 (161.7 °C) | |
| Triple point | 236.8 K (−35.9 °C) ? |
|
| Critical point | 670 K (397 °C) 5.5 MPa |
|
| Enthalpy of fusion | 14.37 kJ/mol | |
| Entropy of fusion | 61.1 J/(mol·K) | |
| Enthalpy of vaporization | 50.6 kJ/mol | |
|
Liquid properties |
||
| ΔfH0liquid | −200.2 kJ/mol | |
| S0liquid | 218 J/(mol·K) | |
| Cp | 159.5 J/(mol·K) | |
| Density | 1.16 ×103 kg/m3 | |
|
Gas properties |
||
| ΔfH0gas | −151.0 kJ/mol | |
| Cp | 90.8 J/(mol·K) | |
|
Safety | ||
| Acute effects | Toxic irritant. Can cause headache, dizziness, nausea, and eventual unconsciousness and death. When inhaled, can cause swelling and spasms of the respiratory tract. | |
| Chronic effecs | Repeated exposure can cause skin allergies and photosensitization. Can cause liver and kidney damage. | |
| Flash point | 62 °C | |
| Autoignition temperature | 315 °C | |
| Explosive limits | 2-19% | |
|
More info | ||
| Properties | NIST WebBook (http://webbook.nist.gov/cgi/cbook.cgi?ID=C98011&Units=SI) | |
| MSDS | Hazardous Chemical Database (http://ull.chemistry.uakron.edu/erd/chemicals1/7/6530.html) | |
|
SI units were used where possible. Unless otherwise stated, standard conditions were used. Disclaimer and references </font> | ||
| Contents |
History
Furfural was first isolated in 1832 by the German chemist Johann Wolfgang Döbereiner, who formed a very small quantity of it as a byproduct of formic acid synthesis. At the time, formic acid was formed by the distillation of dead ants, and Döbereiner's ant bodies probably contained some plant matter. In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of crop materials, including corn, oats, bran, and sawdust, with aqueous sulfuric acid, and he determined that this chemical had an empirical formula of C5H4O2. In 1901, the German chemist Carl Harries deduced furfural's structure.
Except for occasional use in perfume, furfural remained a relatively obscure chemical until 1922, when the Quaker Oats Company began mass-producing it from oat hulls. Today, furfural is still produced from agricultural byproducts.
Properties
Furfural's physical properties are summarized in the table at right. Furfural dissolves readily in most polar organic solvents, but is only slightly soluble in either water or alkanes.
Chemically, furfural participates in the same kinds of reactions as other aldehydes and other aromatic compounds. The aromatic stability of furfural is not as great as in benzene, and furfural participates in hydrogenation and other addition reactions more readily than many other aromatics.
When heated above 250 °C, furfural decomposes into furan and carbon monoxide, sometimes explosively.
When heated in the presence of acids, furfural irreversibly solidifies into a hard thermosetting resin.
Production
Many plant materials contain the polysaccharide hemicellulose, a polymer of sugars containing five carbon atoms each. When heated with sulfuric acid, hemicellulose undergoes hydrolysis to yield these sugars, principally xylose. Under the same conditions of heat and acid, xylose and other five carbon sugars undergo dehydration, losing three water molecules to become furfural.
For crop residue feedstocks, about 10% of the mass of the original plant matter can be recovered as furfural. Furfural and water evaporate together from the reaction mixture, and separate upon condensation.
Uses
Furfural is used as a solvent in petrochemical refining to extract dienes (which are used to make synthetic rubber) from other hydrocarbons.
Furfural, as well as its derivative furfuryl alcohol, can be used either by themselves or in together with phenol, acetone, or urea to make solid resins. Such resins are used in making fiberglass, some aircraft components, and automotive brakes.
Furfural is also used as a chemical intermediate in the production of the solvents furan and tetrahydrofuran.
Safety
When ingested or inhaled, furfural can cause symptoms similar to those of intoxication, including euphoria, headache, dizziness, nausea, and eventual unconsciousness and death due to respiratory failure. Contact with furfural irritates the skin and respiratory tract and can cause the lungs to fill with fluid.
Chronic skin exposure can lead to a skin allergy to the substance, as well as an unusual susceptibility to sunburn. In toxicity studies, furfural has led to tumors, mutations, and liver and kidney damage in animals.de:Furfural