Ether
|
|
- This article is about ether as a general class of chemical compounds. For other meanings, see Ether (disambiguation)
Ether is the general name for a class of chemical compounds which contain an ether group — an oxygen atom connected to two (substituted) alkyl groups. A typical example is the solvent diethyl ether (ethoxyethane, CH3-CH2-O-CH2-CH3).
| Contents |
Similar structures
Structures_not_ethers.png
Ethers are not to be confused with the following classes of compounds with the same general structure R-O-R.
- Aromatic compounds like furan where the oxygen is part of the aromatic system.
- Compounds where one of the carbon atoms next to the oxygen is connected to oxygen, nitrogen, or sulfur:
- Esters R-C(=O)-O-R
- Acetals R-CH(-O-R)-O-R
- Aminals R-CH(-NH-R)-O-R
- Anhydrides R-C(=O)-O-C(=O)-R
Primary, secondary, and tertiary ethers
The terms "primary ether", "secondary ether", and "tertiary ether" are occasionally used and refer to the carbon atom next to the ether oxygen. In a primary ether this carbon is connected to only one other carbon as in diethyl ether CH3-CH2-O-CH2-CH3. An example of a secondary ether is diisopropyl ether (CH3)2CH-O-CH(CH3)2 and that of a tertiary ether is di-tert-butyl ether (CH3)3C-O-C(CH3)3.
Missing image
Dimethylether_chemical_structure.png
Dimethyl ether
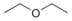 Missing image
Missing image
Diisopropyl_ether_chemical_structure.png
A secondary ether (diisopropyl ether)
Missing image
Di-tert-butyl_ether_chemical_structure.png
A tertiary ether (di-tert-butyl ether)
Dimethyl ether, a primary, a secondary, and a tertiary ether.
Polyethers
Polyethers are polymeric compounds with more than one ether group. Examples are the polymers of ethylene oxide like the crown ethers and polyethylene glycol.
Chemical reactions
Synthesis
- R-OH + R-OH → R-O-R + H2O
- This direct reaction requires drastic conditions (heat and an acid catalyst) and is usually not applicable. Such conditions can destroy the delicate structures of some functional groups. There exist several milder methods to produce ethers.
- R-O- + R-X → R-O-R + X-
- This is called Williamson ether synthesis. It involves treatment of a parent alcohol with a strong base to form the alkoxide anion followed by addition of an appropriate aliphatic compound bearing a suitable leaving group (R-X). Suitable leaving groups (X) include iodide, bromide, or sulfonates. This method does not work if R is aromatic like in bromobenzene. Likewise, this method only gives the best yields for primary carbons, as secondary carbons will undergo E2 elimination on exposure to the basic alkoxide anion used in the reaction.
- R2C=CR2 + R-OH → R2CH-C(-O-R)-R2 (under acid catalysis)
Reactions
Ethers are of very low chemical reactivity. They are hydrolyzed only under drastic conditions like heating with boron tribromide or boiling in hydrobromic acid. Lower mineral acids containing a halogen, such as hydrochloric acid will cleave ethers, but very slowly. Hydrobromic acid and hydroiodic acid are the only two that do so at an appreciable rate.
Ethers can act as Lewis bases. For instance, diethyl ether forms a complex with boron compounds, such as boron trifluoride diethyl etherate F3B:O(CH2CH3)2.
Diethylether_peroxide_chemical_structure.png
Primary and secondary ethers with a CH group next to the ether oxygen easily form highly explosive peroxides (e.g. diethyl ether peroxide) in the presence of oxygen, light, and metal and aldehyde impurities. For this reason ethers like diethyl ether and THF are usually avoided as solvents in industrial processes.
Physical properties
Ether molecules cannot form hydrogen bonds among each other, resulting in a relatively low boiling point comparable to that of the analogous alkanes. Ethers are more hydrophobic than esters or amides of comparable structure.
Nomenclature
In the IUPAC nomenclature system, ethers are named using the general formula "alkoxyalkane", for example CH3-CH2-O-CH3 is methoxyethane. If the ether is part of a more complex molecule, it is described as an alkoxy substituent, so -OCH3 would be considered a "methoxy-" group. The nomenclature of describing the two alkyl groups and appending "ether", e.g. "ethyl methyl ether" in the example above, is a trivial usage.
Important ethers
- Ethylene oxide, the smallest cyclic ether: Missing image
Ethylene_oxide_chemical_structure.png
Chemical structure of ethylene oxide
- Dimethyl ether, an aerosol spray propellant: Missing image
Dimethylether_chemical_structure.png
Chemical structure of dimethyl ether
- Diethyl ether, a common low boiling solvent:

- Dimethoxyethane, a high boiling solvent: Missing image
Dimethoxyethane_chemical_structure.png
Chemical structure of dimethoxyethane
- Dioxane, a cyclic ether and high boiling solvent: Missing image
Dioxane_chemical_structure.png
Chemical structure of dioxane
- THF, a cyclic ether, one of the most polar simple ethers that is used as a solvent: Missing image
THF_chemical_structure.png
Chemical structure of THF
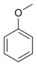
- Anisole (methoxybenzene), a major constituent of the essential oil of anise seed:
- Crown ethers, cyclic polyethers that are used as phase transfer catalysts: Missing image
18-crown-6_chemical_structure.png
Chemical structure of 18-crown-6
- Polyethylene glycol, a linear polyether, e.g. used in cosmetics: Missing image
Polyethylene_glycol_chemical_structure.png
Chemical structure of polyethylene glycol
See also
- Functional group
- Methoxy
- Petroleum ether, not an ether but a low boiling alkane mixture.
- Thioether, analogs of ethers with the oxygen replaced by sulfur.
External links
- ILPI (http://www.ilpi.com/msds/ref/ether.html) page about ethers.da:Æter (funktionel gruppe)
de:Ether eo:etero es:Éter fr:Éther_(chimie) he:אתר (כימיה) it:Eteri ja:エーテル (化学) nl:Ether (chemie) pl:Eter pt:Éter sv:Eter (kemikalie) zh:醚
