Diamond
|
|
- For other uses, see Diamond (disambiguation).
The mineral diamond is a crystalline form, or allotrope, of carbon (other allotropes of carbon include graphite and fullerene). It is one of the most known and most useful of more than 3,000 known minerals. Diamonds are renowned for their superlative physical qualities, especially their hardness—the word "diamond" derives from the ancient Greek adamas (αδάμας; "impossible to tame")—and their high dispersion of light. These properties and others make diamond valued for use in jewelry and a variety of industrial applications. Most diamonds are mined from volcanic pipes, where they have been deposited by deep-origin volcanoes drawing material from over 90 miles (150 km) deep within the Earth, where the pressure and temperature is suitable for diamond formation. Most diamonds are mined in central and southern Africa, although significant deposits have also been discovered in Canada, Russia, Brazil, and Australia. About 130 million carats (26,000 kg) of diamonds are mined annually, with a total value of nearly $9 billion. In addition, nearly four times that mass is artificially produced as synthetic diamond.
The gemological appeal of diamonds lies in their hardness and optical properties. Diamonds used as gems are cut and polished into a number of faceted shapes in order to accentuate these attractive qualities. The hardness of diamonds allows them to hold a polish extremely well and resist scratching (only other diamonds can scratch a diamond), giving excellent luster. The dispersion of white light into a rainbow of colors, known in the trade as fire, is the other primary characteristic of gem diamonds, and has been highly prized throughout history. Gem diamonds are commonly judged by the four Cs: carat, clarity, color, and cut. Diamonds have been treasured as gems since at least 2,500 years ago, when they were used in religious icons in India. Popularity of diamonds as gemstones increased starting in the 19th century as new cutting designs that display diamonds' gem qualities better were developed.
Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools — common applications include the cutting surfaces of saw blades and drill bits, or use of diamond powder as an abrasive. Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most other diamonds, which are excellent resistors. Industrial-grade diamonds are either unsuitable for use as gems or synthetically produced, which lowers their price and makes their use economically feasible. Industrial applications, especially as drill bits and engraving tools, also date to ancient times.
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers (the most important being Antwerp). The De Beers Group, based in Johannesburg, South Africa and London, England, has been the largest player in the diamond industry for over one hundred years; the company and its subsidiaries own mines that produce some 40 percent of annual world diamond production, and control distribution channels handling nearly two thirds of all gem diamonds. Some controversy over diamonds has been generated because of the monopolistic practices historically employed by De Beers including strict control of supply and alleged price manipulation, as well as the practice by some African revolutionary groups of selling conflict diamonds in order to fund their often violent activities.
| Contents |
Material properties
Diamond_unit_cell.PNG
Main article: Material properties of diamond
Diamond is a transparent crystal of pure carbon consisting of tetrahedrally bonded carbon atoms. Humans have been able to adapt diamonds for many uses because of the material's exceptional physical characteristics. Most notable among these properties are the extreme hardness of diamond and its high dispersion index. These two properties form the basis for most modern applications of diamond.
Mechanical properties
Crystal structure: Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Lonsdaleite is a polymorph of diamond (and a distinct mineral species) that crystallizes with hexagonal symmetry; it is rarely found in nature, but is characteristic of synthetic diamonds. A cryptocrystalline variety of diamond is called carbonado. A colorless, grey or black diamond with a tiny radial structure is a spherulite.
The tetrahedral arrangement of atoms in a diamond crystal is the source of many of diamond's properties; graphite, another allotrope of carbon, has a rhombohedral crystal structure and as a result shows dramatically different physical characteristics — contrary to diamond, graphite is a very soft, dark grey opaque mineral.
Hardness: Diamond is the hardest known naturally occurring material, scoring 10 on the relative Mohs scale of mineral hardness and having an absolute hardness value of between 167 and 231 gigapascals in various tests. Diamond's hardness has been known since antiquity, and is the source of its name.
Broad industrial applications of diamond are based on the extraordinary hardness of diamond. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws.
The hardness of diamonds also contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well, keeping its luster over long periods of time. Unlike many other gems, it is well-suited to daily wear due to its resistance to scratching — perhaps contributing to its popularity as the preferred gem in an engagement ring or wedding ring, which are often worn every day.
Toughness: Unlike hardness, which only denotes resistance to scratching, diamond's toughness is only fair to good. Toughness relates to a material's ability to resist breakage from forceful impact. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamonds cut into certain particular shapes are therefore more prone to breakage than others.
Color: Diamonds occur in a variety of transparent hues — colorless, white, steel, blue, yellow, orange, red, green, pink, brown — or colored black. Diamonds with a detectable hue to them are known as colored diamonds. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly-pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice. The most common impurity, nitrogen, causes a yellowish or brownish tinge.
Thermodynamic stability: At surface air pressure (one atmosphere), diamonds are not as stable as graphite, and so the decay of diamond is thermodynamically favorable (δH = −2 kJ / mol). Diamonds will burn at approximately 800 degrees Celsius, providing that enough oxygen is available. This was shown in the late 18th century, and previously described during Roman times. So, despite the popular advertising slogan, diamonds are definitely not forever. However, owing to a very large kinetic energy barrier, diamonds are metastable; under normal conditions, it would take an extreme long time (possibly more than the age of the Universe) to decay into graphite.
Electromagnetic properties
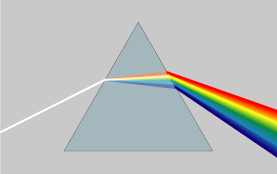
Optical properties: Diamonds exhibit a high dispersion of visible light. This strong ability to split white light into its component colors is an important aspect of diamond's attraction as a gemstone, giving it impressive prismatic action that results in so-called fire in a well-cut stone. The luster of a diamond, a characterization of how light interacts with the surface of a crystal, is brilliant and is described as adamantine, which simply means diamond-like. Some diamonds exhibit fluorescence of various colors under long wave ultraviolet light, but generally show bluish-white, yellowish or greenish fluorescence under X-rays. Some diamonds show no fluorescence.
Electrical properties: Except for most natural blue diamonds which are semiconductors, diamond is a good electrical insulator. Blue diamonds owe their semiconductive property to boron impurities, which act as a doping agent and cause p-type semiconductor behavior. Natural blue diamonds which are not boron-doped, such as those recently recovered from the Argyle diamond mine in Australia that owe their color to an overabundance of hydrogen atoms, are not semiconductors.
Thermal properties: Unlike most electrical insulators, diamond is a good conductor of heat because of the strong covalent bonding within the crystal. Most natural blue diamonds contain boron atoms which replace carbon atoms in the crystal matrix, and also have high thermal conductivity. Specially purified synthetic diamond has the highest thermal conductivity (2000–2500 W/(m·K), five times more than copper) of any known solid at room temperature. Because diamond has such high thermal conductance it is already used in semiconductor manufacture to prevent silicon and other semiconducting materials from overheating.
Media
Diamond_Cubic-F_lattice_animation.gif
Template:Multi-video start Template:Multi-video item Template:Multi-video item Template:Multi-video end
Natural history
Formation
Diamond is formed by prolonged exposure of carbon bearing materials to high pressure and temperature. On Earth, the formation of diamonds is possible because there are regions deep within the Earth that are at a high enough pressure and temperature that the formation of diamonds is thermodynamically favorable (see the diamond phase diagram and geotherms here (http://www.amnh.org/exhibitions/diamonds/formation.html)). Under continental crust, diamonds form starting at depths of about 150 kilometers (90 miles), where pressure is roughly 5 gigapascals and the temperature is around 1200 degrees Celsius (2200 degrees Fahrenheit). Diamond formation under oceanic crust takes place at greater depths due to higher temperatures, which require higher pressure for diamond formation. Long periods of exposure to these high pressures and temperatures allow diamond crystals to grow larger.
Rough_diamond.jpg
Through studies of carbon isotope ratios (similar to the methodology used in carbon dating) except using the stable isotopes C-12 and C-13, it has been shown that the carbon found in diamonds comes from both inorganic and organic sources. Some diamonds, known as harzburgitic, are formed from inorganic carbon originally found deep in the Earth's mantle. In contrast, eclogitic diamonds contain organic carbon from organic detritus that has been pushed down from the surface of the Earth's crust through subduction (see plate tectonics) before transforming into diamond. These two different source carbons have measurably different 13C:12C ratios. Diamonds that have come to the Earth's surface are generally very old, ranging from under 1 billion to 3.3 billion years old.
Diamonds occur most often as euhedral or rounded octahedra and twinned octahedra known as macles. As diamond's crystal structure has a cubic arrangement of the atoms, they have many facets that belong to a cube, octahedron, rhombicosidodecahedron, tetrakis hexahedron or disdyakis dodecahedron. The crystals can have rounded off and unexpressive edges and can be elongated. Sometimes they are found grown together or form double "twinned" crystals grown together at the surfaces of the octahedron. This is all due to conditions in which they form. Diamonds (especially those from secondary deposits) are commonly found coated in nyf, an opaque gum-like skin.
Diamonds can also form in other natural high-pressure high-temperature events. Very small diamonds, known as microdiamonds or nanodiamonds, have been found in impact craters where meteors strike the Earth and create shock zones of high pressure and temperature where diamond formation can occur. Microdiamonds are now used as one indicator of ancient meteorite impact sites. Especially ancient meteorites may contain "star dust", the remnants of dead stars, some of which is composed of extremely tiny diamond crystals.
Surfacing
Diamond-bearing rock is forced close to the surface through deep-origin volcanic eruptions. The magma for such a volcano must originate at a depth where diamonds can be formed, 90 miles (150 km) deep or more (three times or more the depth of source magma for most volcanoes); this is a relatively rare occurrence. Below these typically small surface volcanic craters are formations known as volcanic pipes, which contain material that was pushed toward the surface of the earth by volcanic action, but did not erupt before the volcanic activity ceased. Diamond-bearing volcanic pipes are most commonly found in the oldest regions of continental crust, which relates to the fact that these areas are the coolest portions of the earth's crust, and therefore diamonds can form at the shallowest depths.
The magma in such volcanic pipes is usually one of two characteristic types, which cool into igneous rock known as either kimberlite or lamproite. The magma itself does not contain diamond; instead, it acts as an elevator that carries deep-formed rocks and material upward. These rocks are characteristically rich in magnesium bearing olivine, pyroxene, and amphibole minerals which are usually altered to serpentine under near surface conditions. Certain indicator minerals typically occur within diamondiferous kimberlites and are used as mineralogic tracers in the search for diamond deposits by prospectors. These minerals are rich in chromium (Cr) or titanium (Ti), elements which impart bright colors to the minerals. The most common indicator minerals are chromian garnets (usually bright red Cr-pyrope, and occasionally green ugrandite-series garnets), eclogitic garnets, orange Ti-pyrope, red high chromian spinels, dark chromite, bright green Cr-diopside, glassy green olivine, black picroilmenite, and magnetite. Kimberlite deposits are known as blue ground for the deeper serpentinized part of the deposits, or as yellow ground for the near surface smectite clay and carbonate weathered and oxidized portion.
Once diamonds have been forced to the surface by magma in a volcanic pipe, they may erode out and be distributed over a large area. A volcanic pipe containing diamonds is known as a primary source of diamonds. Secondary sources of diamonds include all areas where a significant number of diamonds, eroded out of their kimberlite or lamproite matrix, accumulate due to water or weather action. These include alluvial deposits and deposits along existing and ancient shorelines, where loose diamonds tend to accumulate due to their approximate size and density. Diamonds have also rarely been found in deposits left behind by glaciers (notably in Wisconsin and Indiana); however, in contrast to alluvial deposits, glacial deposits are not known to be of significant concentration and are therefore not viable commercial sources of diamond.
Diamonds can also be brought to the surface through certain processes which may occur when two continental plates collide forcefully, although this phenomenon is less understood and currently assumed to be uncommon.
Gemological characteristics
The use of diamonds as gemstones of decorative value is the most familiar use to most people today, and is also the earliest use, with decorative use of diamonds stretching back into antiquity. Over time, especially since around 1900, experts in the field of gemology have developed methods of characterizing diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the four Cs, are now commonly used as the basic descriptors of diamonds: these are carat, clarity, color, and cut.
Most gem diamonds are traded on the wholesale market based on single values for each of the four Cs; for example knowing that a diamond is rated as 1.5 carats, VS2 clarity, F color, excellent cut, is enough to reasonably establish an expected price range. More detailed information from within each characteristic can then be used to determine actual market value for individual stones. Consumers who purchase individual diamonds are often advised to use the four Cs to pick the diamond that is "right" for them; to these is sometimes added the "fifth C" of cost.
Other characteristics not described by the four Cs can and do influence the value or appearance of a gem diamond. These characteristics include physical characteristics such as the presence of fluorescence, as well as data on a diamond's history including its source and which gemological institute performed evaluation services on the diamond. Cleanliness also dramatically affects a diamond's beauty.
Carat
The carat weight measures the mass of a diamond. One carat is defined as exactly 200 milligrams (about 0.007 ounce). The point unit — equal to one one-hundredth of a carat (0.01 carat, or 2 mg) — is commonly used for diamonds of less than one carat. All else being equal, the value of a diamond increases exponentially in relation to carat weight, since larger diamonds are both rarer and more desirable for use as gemstones. A review of comparable diamonds available for purchase in March 2005 demonstrates this effect (approximate prices for round cut, G color, VS2 diamonds with "1A" cut grade, as listed on http://www.pricescope.com):
| Carat size | Cost per carat (US$) | Total cost (US$) |
|---|---|---|
| 0.5 carat (50 points) | 3,000 | 1,500 |
| 1.0 carat | 5,000 | 5,000 |
| 1.5 carats | 7,000 | 10,500 |
| 2.0 carats | 10,000 | 20,000 |
| 3.0 carats | 15,000 | 45,000 |
| 5.0 carats | 20,000 | 100,000 |
The price per carat does not increase smoothly with increasing size. Instead, there are sharp jumps around milestone carat weights, as demand is much higher for diamonds weighing just more than a milestone than for those weighing just less. As an example, a 0.95 carat diamond has a significantly lower price per carat than a comparable 1.05 carat diamond, due to differences in demand.
A weekly price list published by Rapaport of New York, of diamond prices per carat, for different diamond cuts, clarity and weights, is currently considered the de-facto retail price baseline. Jewelers often trade diamonds at negotiated discounts off the Rapaport price (e.g., "R -3%").
In the wholesale trade of gem diamonds, carat is often used in denominating lots of diamonds for sale. For example, a buyer may place an order for 100 carats of 0.5 carat, D–F, VS2-SI1, excellent cut diamonds, indicating he wishes to purchase 200 diamonds (100 carats total mass) of those approximate characteristics. Because of this, diamond prices (particularly among wholesalers and other industry professionals) are often quoted per carat, rather than per stone.
Total carat weight (t.c.w.) is a phrase used to describe the total mass of diamonds or other gemstone in a piece of jewelry, when more than one gemstone is used. Diamond solitare earrings, for example, are usually quoted in t.c.w. when placed for sale, indicating the mass of the diamonds in both earrings and not each individual diamond. T.c.w. is also widely used for diamond necklaces, bracelets and other similar jewelry pieces.
Clarity
Main article: Diamond clarity
Clarity is a measure of internal defects of a diamond called inclusions. Inclusions may be crystals of a foreign material or another diamond crystal, or structural imperfections such as tiny cracks that can appear whitish or cloudy. The number, size, color, relative location, orientation, and visibility of inclusions can all affect the relative clarity of a diamond. The Gemological Institute of America (GIA) and others have developed systems to grade clarity, which are generally based on those inclusions which are visible to a trained professional when a diamond is viewed from above under 10x magnification.
Diamonds become increasingly rare when considering higher clarity gradings. Only about 20 percent of all diamonds mined have a clarity rating high enough for the diamond to be considered appropriate for use as a gemstone; the other 80 percent are relegated to industrial use. Of that top 20 percent, a significant portion contains an inclusion or inclusions that are visible to the naked eye upon close inspection. Those that do not have a visible inclusion are known as "eye-clean" and are preferred by most buyers, although visible inclusions can sometimes be hidden under the setting in a piece of jewelry.
Most inclusions present in gem-quality diamonds do not affect the diamonds' performance or structural integrity. However, large clouds can affect a diamond's ability to transmit and scatter light. Large cracks close to or breaking the surface may reduce a diamond's resistance to fracture.
Color
Main article: Diamond color
A chemically pure and structurally perfect diamond is perfectly transparent with no hue, or color. However, in reality almost no gem-sized natural diamonds are absolutely perfect. The color of a diamond may be affected by chemical impurities and/or structural defects in the crystal lattice. Depending on the hue and intensity of a diamond's coloration, a diamond's color can either detract from or enhance its value. For example, most white diamonds are discounted in price as more yellow hue is detectable, while intense pink or blue diamonds (such as the Hope Diamond) can be dramatically more valuable.
Most diamonds used as gemstones are basically transparent with little tint, or white diamonds. The most common impurity, nitrogen, replaces a small proportion of carbon atoms in a diamond's structure and causes a yellowish to brownish tint. This effect is present in almost all white diamonds; in only the rarest diamonds is the coloration due to this effect undetectable. The GIA has developed a rating system for color in white diamonds, from "D" to "Z" (with D being "colorless" and Z having a clear light yellow or brown coloration), which has been widely adopted in the industry and is universally recognized. Diamonds with higher color grades are rarer, in higher demand, and therefore more expensive, than lower color grades. While the prices are higher for colorless diamonds, the exact color most valued by a consumer is a matter of personal preference, with some preferring the very transparent D–F range, while others prefer the "warmer" colors in the G–J range and still others prefer a clearly visible tint.
In contrast to yellow or brown hues, diamonds of other colors are much rarer and more valuable. While even a pale pink or blue hue may increase the value of a diamond, more intense coloration is usually considered more desirable and commands the highest prices. A variety of impurities and structural imperfections cause different colors in diamonds, including yellow, pink, blue, red, green, brown, and other hues. Diamonds with unusual or intense coloration are sometimes labeled "fancy" by the diamond industry. Intense yellow coloration is considered one of the fancy colors, and is separate from the color grades of white diamonds. Gemologists have developed rating systems for fancy colored diamonds, but they are not in common use due to the relative rarity of colored diamonds.
Cut
Main article: Diamond cut
The cut of a diamond describes the manner in which a diamond has been shaped and polished from its beginning form as a rough stone to its final gem proportions. The cut of a diamond describes both the shape a diamond is formed into, as well as the quality of workmanship. Diamond cutting is the art and science of creating a gem-quality diamond out of mined rough.
Shape
Diamonds do not show all of their beauty as rough stones; instead, they must be cut and polished to exhibit the characteristic fire and brilliance that diamond gemstones are known for. Diamonds are cut into a variety of shapes that are generally designed to accentuate these features. The techniques for shaping diamonds have been developed over hundreds of years, with perhaps the greatest achievements made in 1919 by mathematician and gem enthusiast Marcel Tolkowsky. He developed the round brilliant cut by calculating the ideal shape to return and scatter light when a diamond is viewed from above. The modern round brilliant has 57 facets (polished faces), counting 33 on the crown (the top half above the middle or girdle of the stone), and 24 on the pavilion (the lower half below the girdle).
Diamonds which are not cut to the specifications of Tolkowsky's round brilliant shape (or subsequent variations) are known as "fancy cuts." Popular fancy cuts include the baguette (from the French, resembling a loaf of bread), marquise, princess (square outline), heart, briolette (a form of the rose cut), and pear cuts. Generally speaking, these "fancy cuts" are not held to the same strict standards as Tolkowsky-derived round brilliants. Cuts are influenced heavily by fashion: the baguette cut — which accentuates a diamond's luster and downplays its fire — was all the rage during the Art Deco period, whereas the princess cut — which accentuates a diamond's fire rather than its luster — is currently gaining popularity. The princess cut is also popular amongst diamond cutters: of all the cuts, it wastes the least of the original crystal. The past decades have seen the development of new diamond cuts, often based on a modification of an existing cut. Some of these include extra facets. These newly developed cuts are viewed by many as more of an attempt at brand differentiation by diamond sellers, than actual improvements to the state of the art.
Quality
The quality of a diamond's cut is widely considered the most important of the four Cs in determining the beauty of a diamond; indeed, it is commonly acknowledged that a well-cut diamond can appear to be of greater carat weight, and have clarity and color appear to be of better grade than they actually are. The skill with which a diamond is cut determines its ability to reflect and refract light.
In addition to carrying the most importance to a diamond's quality as a gemstone, the cut is also the most difficult to quantitatively judge. A number of factors, including proportion, symmetry, and the relative angles of various facets, are determined by the quality of the cut and can affect the performance of a diamond. A poorly cut diamond with facets cut only a few degrees out of alignment can result in a poorly performing stone. For a round brilliant cut, there is a balance between "brilliance" and "fire." When a diamond is cut for too much "fire," it looks like a cubic zirconia, which gives off much more "fire" than real diamond. A well executed round brilliant cut should reflect most light out from the tabletop and make the diamond appear white when viewed from the top. An inferior cut will produce a stone that appears dark at the center and in some extreme cases the ring settings may show through the top of the diamond as shadows.
Several different theories on the "ideal" proportions of a diamond have been and continue to be advocated by professional gemologists. Recently, there has been a shift away from grading cut by the use of various angles and proportions toward measuring the performance of a cut stone. A number of specially modified viewers have been developed toward this end. One result of this trend is the rise of the phrase "hearts and arrows," describing a characteristic pattern observable on stones exhibiting high symmetry. Hearts and arrows diamonds trade at a 10 percent to 20 percent premium to otherwise comparable diamonds.
The cutting process
Main article: Diamond cutting
The process of shaping a rough diamond into a polished gemstone is both an art and a science. The choice of cut is often decided by the original shape of the rough stone, location of the inclusions and flaws to be eliminated, the preservation of the weight, popularity of certain shapes amongst consumers and many other considerations. The round brilliant cut is preferred when the crystal is an octahedron, as often two stones may be cut from one such crystal. Oddly shaped crystals such as macles are more likely to be cut in a fancy cut—that is, a cut other than the round brilliant—which the particular crystal shape lends itself to.
Even with modern techniques, the cutting and polishing of a diamond crystal always results in a dramatic loss of weight; rarely is it less than 50%. Sometimes the cutters compromise and accept lesser proportions and symmetry in order to avoid inclusions or to preserve the carat rating. Since the per-carat price of diamond shifts around key milestones (such as 1.00 carat), many one-carat diamonds are the result of compromising "Cut" for "Carat." Some jewelry experts advise consumers to buy a 0.99 carat diamond for its better price or buy a 1.10 carat diamond for its better cut, avoiding a 1.00 carat diamond which is more likely to be a poorly cut stone.
Cleaning
Main article: Jewelry cleaning
Although it is not one of the four Cs, cleanliness affects a diamond's beauty as much as any of the four Cs. A clean diamond is more brilliant and fiery than the same diamond when it is "dirty". Dirt or grease on the top of a diamond reduces its luster. Water, dirt, or grease on the bottom of a diamond interferes with the diamond's brilliance and fire. Even a thin film absorbs some light that could have been reflected to the person looking at the diamond. Colored dye or smudges can affect the perceived color of a diamond. Historically, some jewelers' stones were misgraded due to smudges on the girdle, or dye on the culet. Current practice is to thoroughly clean a diamond before grading its color.
Maintaining a clean diamond can sometimes be difficult, as jewelry settings can obstruct cleaning efforts, and oils, grease, and other hydrophobic materials adhere well to a diamond's surface. Some jewelers provide their customers with ammonia-based cleaning kits; ultrasonic cleaners are also popular.
Cleanliness does not affect the diamond's market value, as any competent jeweler will clean the diamond before offering it for sale. However, cleanliness might reflect a diamond's sentimental value: some jewelers have noted a correlation between ring cleanliness and marriage quality [1] (http://www.diamondcuttersintl.com/diamond_education/articles/customers/getting_in_shape.html).
History
Diamonds were first recognized and mined in India, where significant alluvial deposits of the stone could then be found. The earliest written reference can be found in the Sanskrit text Arthasastra (completed around 296 BCE), which describes diamond's hardness, luster, and dispersion. Diamonds quickly became associated with divinity, being used to decorate religious icons, and were believed to bring good fortune to those who carried them. Ownership was restricted among various castes by color, with only kings allowed to own all colors of diamond.
In February 2005, a joint Chinese-US team of archaeologists reported the discovery of four corundum-rich stone ceremonial burial axes originating from China's Liangzhu and Sanxingcun cultures (4000 BCE–2500 BCE) which, due to the axes' specular surfaces, the scientists believe were polished using diamond powder. [2] (http://news.bbc.co.uk/2/hi/science/nature/4555235.stm) [3] (http://www.chinadaily.com.cn/english/doc/2005-02/18/content_417247.htm) Although there are diamond deposits now known to exist close to the burial sites, no direct evidence of coeval diamond mining has been found: the researchers came to their conclusion by polishing corundum using various lapidary abrasives and modern techniques, later comparing the results using an atomic force microscope. At that scale, the surface of the modern diamond-polished corundum most closely resembled that of the axes; however, the polishes of the latter were superior.
Diamonds were traded to both the east and west of India, and were recognized by various cultures for their gemological and industrial uses. The Roman writer Pliny the Elder noted diamond's ornamental uses, as well as its usefulness to engravers due to its hardness, in his work Naturalis Historia. In China, diamonds seem to have been used primarily for engraving jade and drilling holes in beads. Archeological evidence from Yemen suggests that diamonds were used as drill tips as early as the 4th century BCE. In Europe, however, diamonds disappeared for almost 1,000 years following the rise of Christianity due to two effects: early Christians rejected diamonds due to their earlier use in amulets, and Arabic traders restricted the flow of trade between Europe and India.
Diamond_cut_history.png
Until the late Middle Ages, diamonds were most prized in their natural octahedral state, perhaps with the crystal surfaces polished to increase luster and remove foreign material. Around 1300, the flow of diamonds into Europe increased via Venice's trade network, with most flowing through the low country ports of Bruges, Antwerp, and Amsterdam. Also around this time, the taboo against cutting diamonds into gem shapes (established over 1,000 years earlier in the traditions of India) ended, allowing the development of diamond cutting technology to begin in earnest. By 1375, a guild of diamond polishers had been established at Nuremberg. Over the following centuries, various diamond cuts were introduced which increasingly demonstrated the fire and brilliance diamonds are treasured for today: the table cut, the briolette (around 1476), the rose cut (mid 16th century), and by the mid 17th century, the Mazarin, the first brilliant cut diamond design. In 1919, Marcel Tolkowsky determined an ideal round brilliant cut, a design that continues to set the standard for comparison for modern gems. However, the evolution of diamond cuts continues on to this day.
The rise in popularity of diamonds as gems seems to have paralleled increasing availability through European history. In the 13th century, King Louis IX of France established a law that only the king could own diamonds. However, within a century diamonds were popular gems among the moneyed aristocratic and merchant classes, and by at latest 1477 had begun to be used in wedding rings. Popularity continued to rise as new cuts were developed that enhanced the diamond's aesthetic appeal, and has largely continued unabated to this day; diamonds have proven popular with all classes in society as their cost becomes within reach. A number of large diamonds have become historically significant objects, as their inclusion in various sets of crown jewels and the purchase, sale, and sometimes theft of notable diamonds, have sometimes become politicized.
- See also: List of famous diamonds
The diamond industry

The diamond industry can be broadly separated into two basically distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways.
Gem diamond industry
A large trade in gem-grade diamonds exists. Unlike precious metals such as gold or platinum, gem diamonds do not trade as a commodity: there is a substantial mark-up in the sale of diamonds, and there is not a very active market for resale of diamonds. One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to a few locations (most importantly Antwerp, London, Tel Aviv, and increasingly Gujarat), and a single company — De Beers — controls over half of all trade in diamonds.
The De Beers company holds a clearly dominant position in the industry, and has done so since soon after its founding in 1888. De Beers owns or controls a significant portion of the world's rough diamond production facilities (mines) and distribution channels for gem-quality diamonds. At one time it was thought over 80 percent of the world's rough diamonds passed through the Diamond Trading Company (DTC, a subsidiary of De Beers) in London, but presently the figure is estimated at around 60 percent. De Beers has used its monopoly position to establish strict price controls, and aggressively market diamonds directly to consumers in world markets.
The De Beers diamond advertising campaign is acknowledged as one of the most successful and innovative ones in history. N.W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and opened up new markets, even in countries where no diamond tradition had existed before. N.W. Ayer's multifaceted marketing campaign included product placement, advertising the diamond itself rather than the De Beers brand, and building associations with celebrities and royalty. This coordinated campaign has lasted decades and continues today; it is perhaps best captured by the now-familiar slogan "a diamond is forever".
Industrial diamond industry
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20,000 kg annually), unsuitable for use as gemstones and known as bort, are destined for industrial use. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 400 million carats (80,000 kg) of synthetic diamonds are produced annually for industrial use.
The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality except for their small size, can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil), high-performance bearings, and limited use in specialized windows.
With the continuing advances being made in the production of synthetic diamond, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor suitable to build microchips from, or the use of diamond as a heat sink in electronics. Significant research efforts in Japan, Europe, and the United States are under way to capitalize on the potential offered by diamond's unique material properties, combined with increased quality and quantity of supply starting to become available from synthetic diamond manufacturers.
Diamond supply chain
See also: List of diamond mines
The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world. In fact, the amount of power which De Beers has consolidated historically prevented it from direct trade with the United States, as its trade practices led to an indictment for violating antitrust regulations (the case was settled in 2004). The concentration of power only loosens at the retail level, where diamonds are sold by a limited number of distributors, known as sightholders, to jewelers around the world.
Childrenmining_300.jpg
Sources
Historically diamonds were known to be found only in alluvial deposits in southern India; India led the world in diamond production from the time of their discovery in approximately the 9th century BCE to the mid 18th century CE, but the commercial potential of these sources has been exhausted. The first non-Indian diamond source was found in Brazil in 1725. Today, most commercially viable diamond deposits are in Africa, notably in South Africa, Namibia, Botswana, the Republic of the Congo, and Sierra Leone. There are also commercial deposits being actively mined in the Northwest Territories of Canada, Siberia (mostly in Yakutia territory, for example Mir pipe and Udachnaya Pipe), Brazil, and in Northern and Western Australia. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as conflict diamonds or blood diamonds. In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central Africa and west Africa, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002, which is aimed at ensuring that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. The Kimberley Process provides documentation and certification of diamond exports from producing countries to ensure that the proceeds of sale are not being used to fund criminal or revolutionary activities. Although the Kimberly Process has been somewhat successful in limiting the number of conflict diamonds entering the market, conflict diamonds smuggled to market continue to persist to some degree.
Currently, gem production totals nearly 30 million carats (6,000 kg) of cut and polished stones annually, and over 100 million carats (20,000 kg) of diamonds are sold for industrial use each year. In 2003, this constituted total production of nearly $9 billion in value.
Distribution
The Diamond Trading Company, or DTC, is a subsidiary of De Beers and markets rough diamonds produced both by De Beers mines and other mines from which it purchases rough diamond production; in whole, about two thirds of all rough diamonds pass through the company. DTC performs sophisticated sorting of rough diamonds into over 16,000 categories, and then sells bulk lots of rough diamonds to a limited number of sightholders a few times a year.
Once purchased by sightholders, diamonds are cut and polished in preparation for sale as gemstones. The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditionally diamond cutting centers have been Antwerp, Gujarat, Tel Aviv, New York, and Johannesburg. Recently, diamond cutting centers have been established in China and Thailand. Cutting centers with lower costs of labor, notably Gujarat in India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. Demonstrating this, India produces 90% of all cut and polished diamonds by number, but only 55% by value. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems than was previously economically feasible.
Diamonds which have been prepared as gemstones are sold on diamond exchanges called bourses. There are 24 registered diamond bourses. This is the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or as is increasingly popular, sold unset ("loose"). According to the Rio Tinto Group, in 2002 the diamonds produced and released to the market were valued at $9 billion as rough diamonds, $14 billion after being cut and polished, $28 billion in wholesale diamond jewelry, and retail sales of $57 billion. [4] (http://www.riotintodiamonds.com/market/industry.asp)
Synthetics, simulants, and enhancements
Main article: Synthetic diamond
Main article: Diamond simulants
Main article: Diamond enhancement
The gemological and industrial uses of diamond have created a large demand for raw stones. A portion of this demand is now being met by synthetic diamonds, man-made diamonds which have similar properties to natural diamonds. This process has historically produced industrial-grade diamonds, but synthetic diamond producers have recently begun to penetrate the gem diamond market. Diamonds have been manufactured synthetically for over fifty years.
A diamond's gem quality, which is not as dependent on material properties as industrial applications, has invited both imitation and the invention of procedures to enhance the gemological properties of natural diamonds. Materials which have similar gemological characteristics to diamond are known as diamond simulants. The most familiar diamond simulant to most consumers is cubic zirconia (commonly abbreviated as CZ); recently moissanite has also gained cachet as a popular diamond simulant. Both CZ and moissanite are synthetically produced for use as a diamond simulant. Diamond enhancements are specific treatments, performed on natural diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.
Currently, trained gemologists with appropriate equipment are able to distinguish natural diamonds from all synthetic and simulant diamonds, and identify all enhanced natural diamonds. The established natural diamond industry has a vested interest in maintaining the distinction between natural diamonds and other diamonds, and has made significant investments toward that end. However, synthetic diamonds may one day be indistinguishable from natural diamonds, and new techniques for simulants (such as coating them with a very thin diamond-like layer of carbon) are making it harder to easily distinguish between simulants and real diamond.
Symbolism
Mary_of_burgundy.jpg
Because of their extraordinary physical properties, diamonds have been used symbolically since near the time of their first discovery. Perhaps the earliest symbolic use of diamonds was as the eyes of Hindu devotional statues. The diamonds themselves were thought to be endowments from the gods and were therefore cherished. The point at which diamonds began to be associated with divinity is not known, but early texts indicate that it was recognized in India since at least 400 BCE. It is said the Greeks believed diamonds were tears of the gods; the Romans believed they were splinters of fallen stars. Many long dead cultures have sought to explain diamond's superlative properties through divine or mystical affiliations.
In western culture, diamonds are the traditional emblem of fearlessness and virtue, but have also often associated with power, wealth, crime and misfortune. Today, diamonds are used to symbolize eternity and love, being often seen adorning engagement rings and sometimes wedding rings as well. The popularity of this modern tradition can be traced directly to the marketing campaigns of De Beers, starting in 1938. The diamond engagement ring is, however, not an original invention of De Beers. It can be traced to the marriage of Maximilian I (then Archduke of Austria) to Mary of Burgundy in 1477. Other early examples of betrothal jewels incorporating diamonds include the Bridal Crown of Blanche (ca. 1370-1380) and the Heftlein brooch of Vienna (ca. 1430-1440), a pictorial piece depicting a wedding couple. Inaccessibility of diamonds to the vast majority of the population limited the popularity of diamonds as betrothal jewels during this period.
Diamonds were also a symbol of gay community in the 1950s. The Mattachine Society, one of the first and the foremost gay rights groups in the United States, used so-called harlequin diamonds (four smaller diamonds arranged in a pattern to form one larger diamond) as their emblem.
The LifeGem company further taps modern symbolism by offering to synthetically convert the carbonized remains of people or pets into "memorial diamonds." However, many people feel very uncomfortable at the thought of wearing the carbonized remains of people as jewelry.
The diamond is the birthstone for people born in the month of April, and is also used as the symbol of a sixty year anniversary, such as a Diamond Jubilee (see hierarchy of precious substances).
Diamonds are a common focus of fiction. Notable pieces of fiction include Ian Fleming's Diamonds Are Forever (1956) and Neal Stephenson's The Diamond Age (1995). In addition, diamonds are the subject of various myths and legends.
External links
- 3D Interactive Molecular Visualization of Diamond (http://www.sciencetechnologies.com/wikimol/index.php/Diamond) at WikiMol (http://www.sciencetechnologies.com/wikimol) (requires Macromedia Flash)
- Elements vol.1 no.2 (March 2005): Diamonds (http://www.elementsmagazine.org/Elements_online/ELEM_V1n2.pdf) (PDF)
- European Gemological Laboratory USA (http://www.eglusa.com)
- Gemological Institute of America (http://www.gia.edu)
- Interactive structure of bulk diamond (http://newton.ex.ac.uk/research/qsystems/people/sque/diamond/structure.html) (Java applet).
- PBS Nature: Diamonds (http://www.pbs.org/wnet/nature/diamonds/index.html)
- Russian Gemological Server (http://www.cutstudy.com)
- Smithsonian's exhibit of fancy color diamonds (http://www.mnh.si.edu/exhibits/si-gems)
References
- American Museum of Natural History. "The Nature of Diamonds" (http://www.amnh.org/exhibitions/diamonds/). Retrieved March 9, 2005.
- The Columbia Electronic Encyclopedia, Sixth Edition (2003). "Diamond". Retrieved March 9, 2005 at http://www.answers.com/topic/diamond.
- Cuellar, Fred. "Diamonds - Getting Into Shape" (http://www.diamondcuttersintl.com/diamond_education/articles/customers/getting_in_shape.html). Diamond Cutters International. Retrieved April 10, 2005.
- David, Joshua (September 2003). "The New Diamond Age" (http://www.wired.com/wired/archive/11.09/diamond.html). Wired, issue 11.09.
- De Beers Group. "De Beers Group" (http://www.debeersgroup.com/debeersweb). Retrieved March 14, 2005.
- Epstein, Edward Jay (February 1982). "Have You Ever Tried To Sell a Diamond?" (http://www.theatlantic.com/issues/82feb/8202diamond1.htm) (subscription required). The Atlantic Monthly.
- Epstein, Edward Jay (1982). "THE DIAMOND INVENTION" (http://edwardjayepstein.com/diamond/prologue.htm) (Complete book, includes "Chapter 20: Have you ever tried to sell a diamond?")
- Eppler, W.F. Praktische Gemmologie. Rühle-Diebner-Verlag, 1989
- Government of Gujarat (2004). "Vibrant Gujarat: Sector Profiles" (http://www.vibrantgujarat.com/sp-gems.html). Retrieved March 14, 2005.
- Kjarsgaard, B.A. and Levinson, A. A. (2002). Diamonds in Canada. Gems & Gemology, Vol. 38, No. 3, pp. 208–238.
- Pagel - Theisen, Verena. Diamond Grading ABC: the Manual. Rubin & Son, Antwerp, Belgium, 2001. ISBN 3980043460
- Pricescope. "Diamond price report" (http://www.pricescope.com/Reports.asp?shp=8&cut=2). Retrieved March 4, 2005.
- Sque, Steve (March 8, 2005). "Properties of Diamond" (http://newton.ex.ac.uk/research/qsystems/people/sque/diamond/). Retrieved March 10, 2005.
- Tolkowsky, Marcel (1919). Diamond Design: A Study of the Reflection and Refraction of Light in a Diamond. London: E. & F.N. Spon, Ltd. (Web edition (http://www.folds.net/diamond/index.html) as edited by Jasper Paulsen, Seattle, 2001.)
- Tyson, Peter (November 2000). "Diamonds in the Sky" (http://www.pbs.org/wgbh/nova/diamond/sky.html). Retrieved March 10, 2005.
- United Nations Department of Public Information (March 21, 2001). "Conflict Diamonds" (http://www.un.org/peace/africa/Diamond.html). Retrieved March 10, 2005.
- Weiner, K.L., Hochleitner, R., Weiss, S., Voelstadt H. Diamant, Lapis, München, 1994.
- Yarnell, Amanda (February 2, 2004). "The Many Facets of Man-Made Diamonds" (http://pubs.acs.org/cen/coverstory/8205//8205diamonds.html). Chemical & Engineering News, vol. 82, no. 5, pp 26–31.bg:Диамант
cs:Diamant de:Diamant et:Teemant es:Diamante eo:Diamanto fr:Diamant hi:हीरा it:Diamante he:יהלום nl:Diamant ja:ダイヤモンド nb:Diamant nn:Diamant pl:Diament pt:Diamante ru:Алмаз sr:Дијамант sv:Diamant zh:金刚石