Crystal structure
|
|
Sand_Rose_1.jpg
In mineralogy and crystallography, a crystal structure is a unique arrangement of atoms in a crystal. A crystal structure is composed of a unit cell, a set of atoms arranged in a particular way; which is periodically repeated in three dimensions on a lattice. The spacing between unit cells in various directions are called its lattice parameters. The symmetry properties of the crystal are embodied in its space group. A crystal's structure and symmetry play a role in determining many of its properties, such as cleavage, electronic band structure, and optical properties.
| Contents |
Unit cell
A unit cell is a spatial arrangement of atoms which is tiled in three-dimensional space to describe the crystal. The positions of the atoms inside the unit cell are described by the asymmetric unit or basis, the set of atomic positions <math>(x_i, y_i, z_i)<math> measured from a lattice point.
For each crystal structure there is a conventional unit cell, usually chosen to make the resulting lattice as symmetric as possible. However, the conventional unit cell is not always the smallest possible choice. A primitive unit cell of a particular crystal structure is the smallest possible unit cell one can construct such that, when tiled, it completely fills space. A Wigner-Seitz cell is a particular kind of primitive cell which has the same symmetry as the lattice.
Crystal system
The crystal system is the point group of the lattice (the set of rotation and reflection symmetries which leave a lattice point fixed), not including the positions of the atoms in the unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic. Some crystallographers consider the hexagonal crystal system not to be its own crystal system, but instead a part of the trigonal crystal system.
Classification of lattices
| Crystal system | Lattices | |||
| triclinic | Missing image Triclinic.png Triclinic | |||
| monoclinic | simple | centered | ||
| Missing image Monoclinic.png Monoclinic, simple | Missing image Monoclinic-base-centered.png Monoclinic, centered | |||
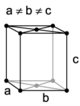
| orthorhombic | simple | base-centered | body-centered | face-centered |
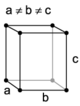
| 
| Missing image Orthorhombic-body-centered.png Orthohombic, body-centered | 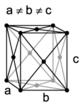
| |
| hexagonal | 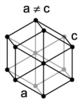
| |||
| rhombohedral (trigonal) | 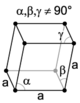
| |||
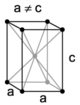
| tetragonal | simple | body-centered | ||
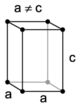
| 
| |||
| cubic (isometric) | simple | body-centered | face-centered | |
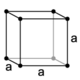
| 
| Missing image Cubic,_face-centered.png Cubic, face-centered | ||
A Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. In three dimensions, there are 14 unique Bravais lattices (distinct from one another in that they have different space groups) in three dimensions. All crystalline materials recognised till now (not including quasicrystals) fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the right.
The crystal structure is one of the lattices with a unit cell, which contains atoms at specific coordinates, at every lattice point. Because it includes the unit cell, the symmetry of the crystal can be more complicated than the symmetry of the lattice.
Point and space groups
The crystallographic point group or crystal class is the set of non-translational symmetries that leave a point in the crystal fixed. There are 32 possible crystal classes.
The space group of the crystal structure is composed of the translational symmetries in addition to the symmetries of the point group. There are 230 distinct space groups.
Defects in crystals
Real crystals feature defects or irregularities in the ideal arrangements described above and it is these defects that critically determine many of the electrical and mechanical properties of real materials. In particular dislocations in the crystal lattice allow shear at much lower stress than that needed for a perfect crystal structure.
See also
- Crystal
- Crystallography
- Crystallographic defect
- Crystal growth
- Liquid crystal
- Cleavage (crystal)
- Seed crystal
For more detailed information in specific technology applications see materials engineering, materials science, ceramics, metallurgy, or materials physics.
External links
- Appendix A from the manual for Atoms, software for XAFS (http://www.planewave.de/icp/atoms/atoms.sgml-7.html)
- Intro to Minerals: Crystal Class and System (http://dave.ucsc.edu/myrtreia/crystal.html)
- Crystal planes and Miller indices (http://www.ece.byu.edu/cleanroom/EW_orientation.phtml)ca:Estructura cristal·lina
de:Kristallsystem fr:Structure cristalline id:struktur kristal it:Sistema cristallino nl:Kristalstructuur ja:結晶構造 pl:Układ krystalograficzny ru:Классификация кристаллических решёток sl:kristalni sistem zh:晶体结构
