Alkane
|
|
An alkane in organic chemistry is a saturated hydrocarbon without cycles, that is, an acyclic hydrocarbon in which the molecule has the maximum possible number of hydrogen atoms and so has no double bonds. Alkanes are also often known as paraffins, or collectively as the paraffin series; these terms, however, are also used to apply only to alkanes whose carbon atoms form a single, unbranched chain; when this is done, branched-chain alkanes are called isoparaffins. Alkanes are aliphatic compounds.
The general formula for alkanes is CnH2n+2; the simplest possible alkane is therefore methane, CH4. The next simplest is ethane, C2H6; the series continues indefinitely. Each carbon atom in an alkane has sp3 hybridization.
| Contents |
Isomerism
The atoms in alkanes with more than three carbon atoms can be arranged in multiple ways, forming different isomers. "Normal" alkanes have a linear, unbranched configuration. The number of isomers increases rapidly with the number of carbon atoms; for alkanes with 1 to 12 carbon atoms, the number of isomers equals 1, 1, 1, 2, 3, 5, 9, 18, 35, 75, 159, and 355, respectively Template:OEIS.
Naming alkanes
IUPAC system
The names of all alkanes end with -ane. Straight-chain alkanes with eight or fewer carbon atoms are named according to the following table, which also gives the name of the alkyl group formed by detaching a terminal hydrogen. For a more complete list, see List of alkanes.
| Alkane name | Alkane formula | Alkyl group | Alkyl group formula |
| methane | CH4 | methyl | CH3 |
| ethane | C2H6 | ethyl | C2H5 |
| propane | C3H8 | propyl | C3H7 |
| butane | C4H10 | butyl | C4H9 |
| pentane | C5H12 | pentyl | C5H11 |
| hexane | C6H14 | hexyl | C6H13 |
| heptane | C7H16 | heptyl | C7H15 |
| octane | C8H18 | octyl | C8H17 |
Branched alkanes are named as follows:
- Identify the longest straight chain of carbon atoms.
- Number the atoms in this chain, starting from 1 at one end and counting upwards to the other end.
- Examine the groups attached to the chain in order and form their names.
- Form the name by looking at the different attached groups, and writing, for each group, the following:
- The number, or numbers, of the carbon atom, or atoms, where it is attached.
- The prefixes di-, tri-, tetra-, etc. if the group is attached in 2, 3, 4, etc. places, or nothing if it is attached in only one place.
- The name of the attached group.
- The formation of the name is finished by writing down the name of the longest straight chain.
To carry out this algorithm, we must know how to name the substituent groups. This is done by the same method, except that instead of the longest chain of carbon atoms, the longest chain starting from the attachment point is used; also, the numbering is done so that the carbon atom next to the attachment point has the number 1.
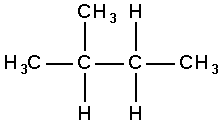
For example, the compound
 is the only 4-carbon alkane possible, apart from butane. Its formal name is 2-methylpropane.
is the only 4-carbon alkane possible, apart from butane. Its formal name is 2-methylpropane.
Pentane, however, has two branched isomers, in addition to its linear, normal form:
Missing image
Dimethylpropane.png
image:dimethylpropane.png
2,2-dimethylpropane
and
The rules presented here are neither unambiguous nor complete. See the article on IUPAC nomenclature for more detail.
Trivial names
Many non-IUPAC, "trivial", or common names are also used:
| Formula | IUPAC name | trivial name |
| C4H10 | Butane | n-butane |
| C5H12 | Pentane | n-pentane |
| C6H14 | Hexane | n-hexane |
| (and so on) | ||
| C4H10 | 2-methylpropane | isobutane i-butane |
| C5H12 | 2-methylbutane | isopentane |
| C6H14 | 2-methylpentane | isohexane |
| (and so on) | ||
| C5H12 | 2,2-dimethylpropane | neopentane |
Properties
Physical properties
- Alkanes are virtually insoluble in water.
- Alkanes are less dense than water.
- Melting points and boiling points of alkanes generally increase with molecular weight and with the length of the main carbon chain.
- At standard conditions, from CH4 to C4H10, alkanes are gaseous; from C5H12 to C17H36, they are liquids; and after C18H38, they are solids.
Chemical properties
- Alkanes have a low reactivity because the C-H and C-C single bonds are relatively stable, difficult to break and non-polar. They do not react with acids, alkalis, metals, or oxidising agents. It may seem surprising, but petrol (octane) has no reaction with concentrated sulphuric acid, sodium metal or potassium manganate. This inertness is the source of the term paraffins (Latin para+affinis, with the meaning here of "lacking affinity").
Reactions
Cracking reactions
"Cracking" breaks larger molecules into smaller ones. This can be done with a thermic or catalytic method. The thermal cracking process follows a homolytic mechanism, that is, bonds break symmetrically and thus pairs of free radicals are formed. The catalytic cracking process involves the presence of acid catalysts (usually solid acids such as silica-alumina and zeolites) which promote a heterolytic (asymmetric) breakage of bonds yielding pairs of ions of opposite charges, usually a carbocation and the very unstable hydride anion. Carbon-localized free radicals and cations are both highly unstable and undergo processes of chain rearrangement, C-C scission in position beta (i.e., cracking) and intra- and intermolecular hydrogen transfer or hydride transfer. In both types of processes, the corresponding reactive intermediates (radicals, ions) are permanently regenerated, and thus they proceed by a self-propagating chain mechanism. The chain of reactions is eventually terminated by radical or ion recombination.
Here is an example of cracking with butane CH3-CH2-CH2-CH3
- 1st possibility (48%): breaking is done on the CH3-CH2 bond.
CH3* / *CH2-CH2-CH3
after a certain number of steps, we will obtain an alkane and an alkene: CH4 + CH2=CH-CH3
- 2nd possibility (38%): breaking is done on the CH2-CH2 bond.
CH3-CH2* / *CH2-CH3
after a certain number of steps, we will obtain an alkane and an alkene from different types: CH3-CH3 + CH2=CH2
- 3rd possibility (14%): breaking of a C-H bond
after a certain number of steps, we will obtain an alkene and hydrogen gas: CH2=CH-CH2-CH3 + H2
Halogenation reaction
R + X2 → RX + HX
These are the steps when methane is chlorinated. This is a highly exothermic reaction that can lead to an explosion.
1. Initiation step: splitting of a chlorine molecule to form two chlorine atoms. A chlorine atom has an unpaired electron and acts as a free radical.
Cl2 → Cl* / *Cl
energy provided by UV.
2. Propagation (two steps): a hydrogen atom is pulled off from methane then the methyl pulls a Cl from Cl2
CH4 + Cl* → CH3* + HCl
CH3* + Cl2 → CH3Cl + Cl*
This results in the desired product plus another Chlorine radical. This radical will then go on to take part in another propagation reaction causing a chain reaction. If there is an excess of Chlorine, other products like CH2Cl2 may be formed.
3. Termination step: recombination of two free radicals
- Cl* + Cl* → Cl2, or
- CH3* + Cl* → CH3Cl, or
- CH3* + CH3* → C2H6.
The last possibilty in the termination step will result in an impurity in the final mixture; notably this results in an organic molecule with a longer carbon chain than the reactants.
Combustion
R + O2 → CO2 + H2O + H2
Combustion is a very exothermic reaction. If the quantity of O2 is insufficient, it will form a poison called carbon monoxide (CO). Here is an example with methane:
CH4 + 2 O2 → CO2 + 2 H2O
with less O2:
2 CH4 + 3 O2 → 2 CO + 4 H2O
with even less O2:
CH4 + O2 → C + 2 H2O
See also
ca:Alcà da:Alkan de:Alkane es:Alcano eo:Alkano fr:Alcane he:אלקאן it:Alcani nl:Alkaan ja:アルカン no:Alkan nn:Alkan pl:Alkan pt:Alcano ru:Алканы sk:Alkán fi:Alkaani su:alkana sv:Alkan zh:烷烃