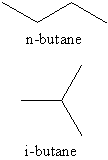Butane
|
|
| General | |
|---|---|
| Chemical Name | Butane n-butane |
| Chemical formula | CH3(CH2)2CH3 |
| Molecular weight | 58.1 g/mol |
| CAS number | 106-97-8 |
| MSDS | Butane MSDS |
| Physical properties | |
| pH (10% solution with water) | 7.0 |
| Phase behavior | |
| Melting point | -138.3 °C (134.9 K) |
| Boiling point | -0.5 °C (272.7 K) |
| Heat of vaporization (ΔvapH) | 21 kJ/mol |
| Safety | |
| Flash point | -60 °C |
| Precautions | |
| |
| Gas properties | |
| ΔfH0gas | -126 kJ/mol |
| S0gas | 310 J/mol·K |
| Cp | 97 J/mol·K |
| General | |
| Chemical Name | 2-methylpropane Isobutane |
| Chemical formula | CH3CH(CH3)2 |
| Molecular weight | 58.1 g/mol |
| Phase behavior | |
| Melting point | -159.6 °C (113.6 K) |
| Boiling point | -11.7 °C (261.5 K) |
|
Except where noted, all data was produced under conditions of standard temperature and pressure. | |
Butane, also called n-butane, is the unbranched alkane with four carbon atoms, CH3CH2CH2CH3. Butane is also used as a collective term for n-butane together with its only other isomer, iso-butane (also called i-butane, isobutane, or 2-methylpropane), CH3CH(CH3)2.
Butanes are highly flammable, colorless, easily liquefied gases.
| Contents |
Chemical structure
n-Butane has the following chemical structure:
H H H H
| | | |
H - C - C - C - C - H
| | | |
H H H H
Isobutane, on the other hand, has a branched-chain structure:
Reactions and uses
When air is plentiful, butane burns to form carbon dioxide and steam:
- butane + oxygen -> carbon dioxide + steam
When air is limited, carbon (soot) or carbon monoxide may also be formed.
Butane gas is sold bottled as a fuel for cooking and camping, in which case it is referred to commercially as LPG, or, in the UK, calor gas. It is also used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays.
Recent concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers. When used as a refrigerant, isobutane is also known as R600a.
See also
External links
- n-Butane (http://www.it.swin.edu.au/personal/fwang/Mom/Mom_butane.html), Molecule of the Month (http://www.chm.bris.ac.uk/motm/motm.htm).
| Alkanes | |||||||||||||||||||||||||||||||
|
methane |
| |
ethane |
| |
propane |
| |
butane |
| |
pentane |
| |
hexane | |||||||||||||||||||||
|
heptane |
| |
octane |
| |
nonane |
| |
decane |
| |
undecane |
| |
dodecane |
| ||||||||||||||||||||
|
tridecane |
| |
tetradecane |
| |
pentadecane |
| |
hexadecane |
| |
heptadecane |
| |
octadecane |
| ||||||||||||||||||||
|
nonadecane |
| |
eicosane |
| |
heneicosane |
| |
docosane |
| |
tricosane |
| |
tetracosane |
| ||||||||||||||||||||
|
pentacosane |
| |
hexacosane |
| |
heptacosane |
| |
octacosane |
| |
nonacosane |
| |
triacontane |
| ||||||||||||||||||||
|
hentriacontane |
| |
dotriacontane |
| |
tritriacontane |
| |
tetratriacontane |
| |
pentatriacontane |
| |
hexatriacontane |
| ||||||||||||||||||||
de:Butan es:Butano eo:Butano fr:Butane it:Butano ko:부탄 (물질) nl:Butaan ja:ブタン pt:Butano ru:Бутан (вещество) sl:Butan (plin) sv:Butan