Prostaglandin
|
|
A prostaglandin is any member of a group of lipid compounds that are derived from fatty acids and have important functions in the animal body. Every prostaglandin contains 20 carbon atoms, including a 5 carbon ring. They are mediators and have a variety of strong physiological effects; although they are technically hormones they are rarely classified as such.
The prostaglandins together with the thromboxanes form the prostanoid class of fatty acid derivatives; the prostanoid class is a subclass of eicosanoids.
| Contents |
History and name
The name prostaglandin comes from the prostate gland. When prostaglandin was first isolated from seminal fluid in 1936, it was believed to be part of the prostatic secretions. In 1971, it was determined that aspirin-like drugs could inhibit the synthesis of prostaglandins. The biochemists Sune K. Bergström, Bengt I. Samuelsson and John R. Vane jointly received the 1982 Nobel Prize in Physiology or Medicine for their research on prostaglandins.
Biochemistry
Biosynthesis
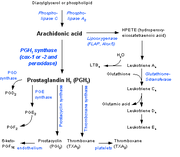
Prostaglandins are found in virtually all tissues and organs. They are autocrine and paracrine lipid mediators which act upon platelet, endothelium, uterine and mast cells among others. They are synthesized in the cell from arachidonic acid created by phospholipase A2. The intermediate is then passed into one of either the cyclooxygenase or lipoxygenase pathways to form either prostaglandin and thromboxane or leukotriene. The cyclooxygenase pathway produces thromboxane, prostacyclin and prostaglandin D, E and F. The lipoxygenase pathway is active in leukocytes and in macrophages and synthesises leukotrienes. Prostaglandins are released through the prostaglandin transporter on the cell's plasma membrane.Cyclooxygenases
Prostaglandins are produced from arachidonic acid by cyclooxygenases (COX-1 and COX-2). COX-1 is responsible for the baseline levels of prostaglandins while COX-2 produces prostaglandins through stimulation. While COX-1 is located in the blood vessels, stomach and the kidneys, prostaglandin level are induced by COX-2 in scenarios of inflammation.
Function
There are currently nine known receptors of prostaglandins on various cell types. Prostaglandins thus act on a variety of cells such as vascular smooth muscle cells causing constriction or dilation, on platelets causing aggregation or disaggregation and on spinal neurons causing pain. Prostaglandins act principally on a subfamily of G protein coupled receptors. Most of these GPCRs are located at the periphery of target cells at the plasma membrane, however, a few exist within the cell at the nuclear envelope. They may also act upon peroxisome proliferator-activated receptors. Prostaglandins have a wide variety of actions but most cause muscular constriction and mediate inflammation. Other effects include calcium movement, hormone regulation and cell growth control. Thromboxane is created in platelets and causes vascular constriction and platelet aggregation. Prostacyclin comes from cells in the blood vessel walls and is antagonistic to thromboxane.
Prostaglandins are potent but have a short half-life before being inactivated and excreted. Therefore, they only exert a paracrine (locally active) function.
15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) is a PGD2 derivative that acts on PPAR intracellular receptors.
Role in pharmacology
NSAIDs inhibit cyclooxygenase and reduce prostaglandin synthesis. Corticosteroids inhibit phospholipase A2 production by boosting production of lipocortin, an inhibitor protein. Relatively new drugs, known as COX-2 selective inhibitors or coxibs, are used as specific inhibitors of COX-2. The development of these drugs allowed the circumvention of the negative gastrointestinal effects while effectively reducing inflammation. However, recently it has been shown that these drugs raise the risk of myocardial infarction, therefore they are only limitedly used now.
Synthetic prostaglandins are used:
- to induce childbirth (PGE2 or PGF2, with mifepristone);
- to prevent closure of ductus arteriosus in newborns with particular congenital heart defects;
- to prevent and treat peptic ulcers (PGE);
- as a vasodilator in severe Raynaud's phenomenon or ischemia of a limb;
- in pulmonary hypertension
- in treatment of glaucoma (as in bimatoprost ophthalmic solution, a synthetic prostamide analog with ocular hypotensive activity).de:Prostaglandin
es:Prostaglandina fr:Prostaglandine nl:Prostaglandine ja:プロスタグランジン zh:前列腺素