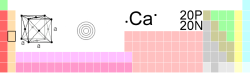
Calcium
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | calcium, Ca, 20 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Series | alkaline earth metal | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 2 (IIA), 4, s | ||||||||||||||||||||||||||||||||||||||||||||||||
| Density, Hardness | 1550 kg/m3, 1.75 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white Missing image Ca,20.jpg | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic weight | 40.078 amu | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 180 (194) pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 174 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| van der Waals radius | no information | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar]4s2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| e- 's per energy level | 2, 8, 8, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states (Oxide) | 2 (strong base) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic face centered | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State of matter | solid (paramagnetic) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1115 K (1548?F) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1757 K (2703?F) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 26.20 ×10-6 m3/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 153.6 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 8.54 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | 254 Pa at 1112 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 3810 m/s at 293.15 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.00 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 632 J/(kg*K) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical conductivity | 29.8 106/(m·ohm) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 201 W/(m*K) | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1st ionization potential | 589.8 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd ionization potential | 1145.4 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd ionization potential | 4912.4 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||||||||||||||||||||||||||||||||
Calcium is a chemical element in the periodic table that has the symbol Ca and atomic number 20. Calcium is a soft grey alkaline earth metal that is used as a reducing agent in the extraction of thorium, zirconium and uranium. This element is also the fifth most abundant element in the earth's crust. It is essential for living organisms, particularly in cell physiology.
| Contents |
Notable characteristics
Calcium is a rather soft metallic element that is purified by electrolysis from calcium fluoride. It burns with a yellow-red flame and forms a white nitride coating when exposed to air. It reacts with water displacing hydrogen and forming calcium hydroxide.
Applications
Calcium is an important component of a healthy diet. Its minor deficit can affect bone and teeth formation. Its excess can lead to kidney stones. Vitamin D is needed to absorb calcium. Dairy products have large levels of calcium.
For more information about Ca in living nature, see calcium in biology.
Other uses include:
- Reducing agent in the extraction of other metals such as uranium, zirconium, and thorium.
- Deoxidizer, desulfurizer, or decarburizer for various ferrous and nonferrous alloys.
- Alloying agent used in the production of aluminium, beryllium, copper, lead, and magnesium alloys.
- It is also used in making cements and mortar that are used in building.
Compounds
Quicklime (CaO) is used in many chemical refinery processes and is made by heating and carefully adding water to limestone. When CaO is mixed with sand it hardens into a mortar and is turned into plaster by carbon dioxide uptake. Mixed with other compounds, CaO forms an important part of Portland cement.
When water percolates through limestone or other soluble carbonate rocks, it partially disolves part of the rock and causes cave formation and characteristic stalactites and stalagmites and also forms hard water. Other important calcium compounds are nitrate, sulfide, chloride, carbide, cyanamide, and hypochlorite.
Isotopes
Calcium has six stable isotopes, two of which occur in nature: stable Ca-40 and radioactive Ca-41 with a half-life = 103,000 years. 97% of the element is in the form of Ca-40. Ca-40 is one of the daughter products of K-40 decay, along with Ar-40. While K-Ar dating has been used extensively in the geological sciences, the prevalence of Ca-40 in nature has impeded its use in dating. Techniques using mass spectrometry and a double spike isotope dilution have been used for K-Ca age dating. Unlike cosmogenic isotopes that are produced in the atmosphere, Ca-41 is produced by neutron activation of Ca-40. Most of its production is in the upper metre or so of the soil column where the cosmogenic neutron flux is still sufficiently strong. Ca-41 has received much attention in stellar studies because Ca-41 decays to K-41, a critical indicator of solar-system anomalies.
See also: disorders of calcium metabolism