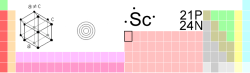
Scandium
|
|
| |||||||||||||||||||
| General | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | scandium, Sc, 21 | ||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||
| Group, Period, Block | 3, 4, d | ||||||||||||||||||
| Density, Hardness | 2985 kg/m3, U/K | ||||||||||||||||||
| Appearance | Silvery white Missing image Sc,21.jpg | ||||||||||||||||||
| Atomic properties | |||||||||||||||||||
| Atomic weight | 44.955910 amu | ||||||||||||||||||
| Atomic radius (calc.) | 160 (184) pm | ||||||||||||||||||
| Covalent radius | 144 pm | ||||||||||||||||||
| van der Waals radius | no data | ||||||||||||||||||
| Electron configuration | [Ar]3d1 4s2 | ||||||||||||||||||
| e- 's per energy level | 2, 8, 9, 2 | ||||||||||||||||||
| Oxidation states (Oxide) | 3 (weak base) | ||||||||||||||||||
| Crystal structure | Hexagonal | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| State of matter | Solid (__) | ||||||||||||||||||
| Melting point | 1814 K (2806 ?F) | ||||||||||||||||||
| Boiling point | 3103 K (5126 ?F) | ||||||||||||||||||
| Molar volume | 15.00 ×10-6 m3/mol | ||||||||||||||||||
| Heat of vaporization | 314.2 kJ/mol | ||||||||||||||||||
| Heat of fusion | 14.1 kJ/mol | ||||||||||||||||||
| Vapor pressure | 22.1 Pa at 1812 K | ||||||||||||||||||
| Speed of sound | no data m/s at 293.15 K | ||||||||||||||||||
| Miscellaneous | |||||||||||||||||||
| Electronegativity | 1.36 (Pauling scale) | ||||||||||||||||||
| Specific heat capacity | 568 J/(kg*K) | ||||||||||||||||||
| Electrical conductivity | 1.77 106/(m·ohm) | ||||||||||||||||||
| Thermal conductivity | 15.8 W/(m*K) | ||||||||||||||||||
| 1st ionization potential | 633.1 kJ/mol | ||||||||||||||||||
| 2nd ionization potential | 1235.0 kJ/mol | ||||||||||||||||||
| 3rd ionization potential | 2388.6 kJ/mol | ||||||||||||||||||
| 4th ionization potential | 7090.6 kJ/mol | ||||||||||||||||||
| 5th ionization potential | 8843 kJ/mol | ||||||||||||||||||
| 6th ionization potential | 10679 kJ/mol | ||||||||||||||||||
| 7th ionization potential | 13310 kJ/mol | ||||||||||||||||||
| 8th ionization potential | 15250 kJ/mol | ||||||||||||||||||
| 9th ionization potential | 17370 kJ/mol | ||||||||||||||||||
| 10th ionization potential | 21726 kJ/mol | ||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||
| |||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||
Scandium is a chemical element in the periodic table that has the symbol Sc and atomic number 21. A soft, silvery, white transition element, scandium occurs in rare minerals from Scandinavia and it is sometimes classified along with yttrium and the lanthanides as a rare earth.
| Contents |
Notable characteristics
Scandium is a rare, soft, silvery, trivalent, very light metallic element that develops a slightly yellowish or pinkish cast when exposed to air. This element resembles yttrium and rare earth metals more than it resembles aluminium or titanium (which are closer on the periodic table). The most common oxidation state of scandium is +3 and this metal is not attacked by a 1:1 mixture of HNO3 and 48% HF.
Applications
Approximately 20 kg (as Sc2O3) of scandium are used annually in the United States to make high-intensity lights. Scandium iodide added to mercury-vapor lamps produces a highly efficient artificial light source that resembles sunlight and allows good color reproduction with TV cameras. About 80 kg of scandium is used in lightbulbs globally per year. The radioactive isotope Sc-46 is used in oil refinery crackers as a tracing agent. The main application by volume is in aluminium-scandium alloys for the aerospace industry and for sports equipment (bikes, baseball bats, etc.) which rely of high performance materials. When added to aluminium, scandium can produce improvements in strength (at ambient and elevated temperature), ductility, aging response and grain refinement through the formation of the Al3Sc phase. Furthermore, it has been shown to reduce solidification cracking during the welding of high strength Al alloys.
History
Dmitri Mendeleev used his periodic law, in 1869, to predict the existence and some properties of three unknown elements including one he called ekaboron .
Lars Fredrick Nilson and his team, apparently unaware of that prediction in the spring of 1879, were looking for rare earth metals; using spectrum analysis he found a new element within the minerals euxenite and gadolinite. He named it Scandium, from the Latin Scandia meaning "Scandinavia", and by way of isolating the element he processed 10 kilograms of euxenite with other rare-earth residues, obtaining about 2 grams of very pure scandium oxide (Sc2O3).
Per Teodor Cleve concluded that scandium corresponded well to the hoped-for ekaboron, and notified Mendeleev of this in August.
Metallic scandium was prepared for the first time in 1937, by electrolysis of a eutectic melt of potassium, lithium, and scandium chlorides at 700 to 800° C. Tungsten wire in a pool of liquid zinc were the electrodes in a graphite crucible. The first pound of 99% pure scandium metal wasn't produced until 1960.
Occurrence
Rare minerals from Scandinavia and Malagasy such as thortveitite, euxenite and gadolinite are the only known concentrated sources of this element (which is never found as a free metal).
Element 21 is the 23rd most abundant element in the sun and similar stars but on earth it is only the 50th most abundant element. Scandium is distributed widely on earth, occurring in trace quantities in over 800 minerals. The blue color of the aquamarine variety of beryl is thought to be caused by scandium. It is an important part of the rare mineral thortveitite and is found in residues that remain after tungsten is extracted from Zinnwald wolframite.
Thortveitite is the primary source of scandium with uranium mill tailings by-products also being an important source. Pure scandium is commercially produced by reducing scandium fluoride with calcium metal.
The main source source of scandium is from military stockpiles from the former Soviet Union, which were themselves extracted from uranium tailings. There is no primary production in the Americas or Europe.
Isotopes
Naturally occurring scandium is composed of 1 stable isotope Sc-45. 13 radioisotopes have been characterized with the most stable being Sc-46 with a half-life of 83.79 days, Sc-47 with a half-life of 3.3492 days, and Sc-48 with a half-life of 43.67 hours. All of the remaining radioactive isotopes have half-lifes that are less than 4 hours and the majority of these have half lifes that are less than 2 minutes. This element also has 5 meta states with the most stable being Scm-44 (t? 58.6 h).
The isotopes of scandium range in atomic weight from 39.978 amu (Sc-40) to 53.963 amu (Sc-54). The primary decay mode before the only stable isotope, Sc-45, is electron capture and the primary mode after is beta emission. The primary decay products before Sc-45 are element 20 (calcium) isotopes and the primary products after are element 22 (titanium) isotopes.
Precautions
Scandium metal powder is combustible and presents a fire hazard.
Template:Chem clipart
References
- Los Alamos National Laboratory – Scandium (http://pearl1.lanl.gov/periodic/elements/21.html)