Acetylcholine
|
|
The chemical compound acetylcholine, often abbreviated as ACh, was the first neurotransmitter to be identified. It is a chemical transmitter in the central nervous system (CNS) as well as in the parasympathetic nervous system in many organisms including humans.
| Contents |
Chemistry
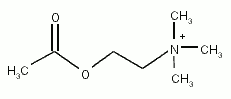
Acetylcholine is an ester of acetic acid and choline with chemical formula CH3COOCH2CH2N+(CH3)3 and structure:
This structure is reflected in the systematic name, 2-(acetyloxy)-N,N,N-trimethylethanaminium.
When it binds to acetylcholine receptors of striated muscle fibers, it stimulates those fibers to contract. Acetylcholine is also used in the brain, where it tends to cause excitatory actions. The glands that receive impulses from the parasympathetic part of the autonomic nervous system are also stimulated in the same way. This is why an increase in acetylcholine causes a decreased heart rate and increased production of saliva.
Acetylcholine is synthesized in certain neurons by the enzyme choline acetyltransferase from the compounds choline and acetyl-CoA. Organic mercurial compounds have a high affinity for sulfhydryl groups, which attributes to its effect on enzyme dysfunction of choline acetyl transferase. This inhibition may lead to acetylcholine deficiency, contributing to the signs and symptoms of motor dysfunction.
Normally, the acetylcholine is quickly removed after having performed its action; this is done by the enzyme acetylcholinesterase which converts acetylcholine into choline and acetate. The devastating effects of nerve agents are due to their inhibition of this enzyme, resulting in continuing stimulation of the muscles, glands and central nervous system. Certain insecticides are effective because they inhibit this enzyme in insects. On the other hand, since a shortage of acetylcholine in the brain has been associated with Alzheimer's disease, some drugs that inhibit acetylcholinesterase are used in the treatment of that disease.
Botulin acts by suppressing the release of acetylcholine. Nicotine acts by increasing the activity of certain acetylcholine receptors, as does muscarine. Conversely, atropine and scopolamine act by blocking these receptors. Atropine and scopolamine are anticholinergic agents.
The disease myasthenia gravis, characterized by muscle weakness and fatigue, occurs when the body inappropriately produces antibodies against acetylcholine receptors, and thus inhibits proper acetylcholine signal transmission. Drugs which competitively inhibit acetylcholinesterase (e.g., neostigmine or physostigmine) are effective in treating this disorder.
Acetylcholine was first identified in 1914 by Henry Hallett Dale, then confirmed as a neurotransmitter by Otto Loewi. For their work, they received the 1936 Nobel Prize in Physiology or Medicine.
Release sites
Acetylcholine is released by:
- pre- and post- ganglionic parasympathetic neurons
- preganglionic sympathetic neurons (and also postganglionic sudomotor neurons, i.e. the ones that control sweating)
- somatic neurons (the ones that control sensation and muscle contraction)
- some CNS neurons
Pharmacology
Acetylcholine is sometimes used during cataract surgery to produce rapid constriction of the pupil. It must be administered intraocularly because corneal cholinesterase metabolizes topically administered ACh before it can diffuse into the eye. It is sold by the trade name Miochol-E (CIBA Vision).
Blocking or mimicking the action of acetylcholine has many uses in medicine. Cholinesterase inhibitors increase the action of acetylcholine by delaying its degradation; some have been used as nerve poisons or pesticides. Clinically they are used to reverse the action of muscle relaxants, to treat myasthenia gravis and in Alzheimer's disease (rivastigmine, which increases cholinergic activity in the brain).
Atropine suppresses nicotinergic activity more than cholinergic activity, and is therefore a parasympatholytic (it abolishes the activity of the parasympathetic nervous system). Similar drugs are used to induce mydriasis (dilation of the pupil), in cardiopulmonary resuscitation and many other situations.
Sources
- Brenner, G. M. (2000). Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6
- Canadian Pharmacists Association (2000). Compendium of Pharmaceuticals and Specialties (25th ed.). Toronto, ON: Webcom. ISBN 0-919115-76-4
External links
- http://www.neurosci.pharm.utoledo.edu/MBC3320/acetylcholine.htm
- http://www.neuro.wustl.edu/neuromuscular/mother/acetylcholine.htmde:Acetylcholin
fr:Acétylcholine he:אצטילכולין nl:Acetylcholine ja:アセチルコリン pl:Acetylocholina ru:Ацетилхолин tl:Acetylcholine