Titration
|
|
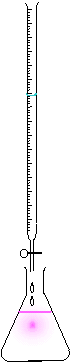
Titration is a standard laboratory method of chemical analysis which can be used to determine the concentration of a known reactant.
A reagent, called the titrant, of known concentration and volume is used to react with a measured volume of reactant. Using a calibrated burette to add the titrant, it is possible to determine the exact amount that has been consumed when the endpoint is reached. The endpoint of a titration is when the pH of the reactant is just about equal to 7, and when the reactant stops reverting back to its original color.
Many methods can be used to indicate the endpoint of a reaction; titrations often use visual indicators (the reactant mixture changes colour). In simple acid-base titrations a pH indicator may be used, such as phenolphthalein, which turns (and stays) pink when a certain pH is reached or exceeded. Due to the logarithmic nature of the pH curve, the transitions are generally extremely sharp, and thus a single drop of titrant just before the endpoint can increase the pH by several points - leading to an immediate colour change in the indicator.
| Contents |
Procedure
N.B. Before starting, make sure that all of your glassware—especially the burette—is clean and dry.
- Accurately measure a volume of the reactant into to a beaker or Erlenmeyer flask.
- Add a suitable indicator to the flask.
- Pour the titrant into the burette, read the start-point of the liquid on the burette.
- Turn the tap of the burette to allow the titrant to slowly fall into the reactant. Swirl the flask with the other hand or with a magnetic "flea".
- The indicator should change colour as the titrant is added, but then quickly return to its original colour.
- As the end-point is approached, the indicator takes longer to turn back to its starting colour. Add the titrant more slowly at this point (one drop at a time).
- When the indicator remains at its end colour, the reaction has reached the end point. Measure the amount of titrant liquid used, as shown on the scale of the burette.
- Repeat as many trials as needed, then average the volumes.
Once the number of moles of reactant that have been neutralised has been determined then it is easy to calculate the concentration in moles per litre.
<math>C_r=\frac{N_r}{V_r}<math>
in the above formula
C represents the concentration
N represents the number of moles
V represents the volume of the solution
Biodiesel
As applied to biodiesel, titration is the act of determining the acidity of a sample of WVO by the dropwise addition of a known base to the sample while testing with pH paper for the desired neutral pH=7 reading. By knowing how much base neutralizes an amount of WVO, we discern how much base to add to the entire batch.
Types
Different types of titration include:
- Acid-base titration
- Redox titration is a type of titration based on a redox reaction between the analyte and titrant.
- Precipitation
- Complexometric titration is a type of titration based on complex formation between the analyte and titrant.
- Conductometry uses the change of conductivity of the solution
- Isothermal titration calorimeter uses the heat of reaction in an
- Spectroscopy measures the absorption of either photons or electrons.
- Back titrations are like normal titrations, except that a known excess of a standard reagent is added to the solution being titrated. The solution is then titrated back, taking into account the addition of the excess. Back titrations are useful if the end point of the reverse titration is easier to identify than the end point of the normal titration.
- Virus titration to determine the virus concentration. May be based on TCID50, EID50, ELD50, LD50 or pfu.
Although the vast majority of titrations are carried out in aqueous solution, other solvents such as glacial acetic acid, are used for special purposes.
The word "titration" comes from the Latin "titalus," meaning inscription or title. The French word, titre, also comes from this origin, meaning rank. Titration is by definition the determination of rank or concentration of a solution so it corresponds.
See also
de:Titration es:Titración it:Titolazione nl:Titratie ja:滴定 sv:Titrering
