Acid-base titration
|
|
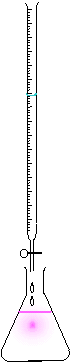
An acid-base titration is a method in chemistry that allows quantitative analysis of the concentration of an unknown acid or base solution. It makes use of the neutralization reaction that occurs between acids and bases, and that we know how acids and bases will react if we know their formula.
| Contents |
Equipment
The key equipment used in a titration are:
- Burette
- Pipette
- Acid/Base Indicator (the one used varies depending on the reactants)
- Conical Flask
- Standard Solution (a solution of known concentration, a common one is aqueous Na2CO3)
- Solution of unknown concentration
Method
The method is as follows.
Before starting the titration we must choose a suitable indicator. The end point of the reaction, when all the products have reacted, will have a pH dependant on the relative strengths of the acids and bases. We can determine roughly the pH of the end point using the following rules:
- A strong acid reacts with a strong base to form a neutral (pH=7) solution.
- A strong acid reacts with a weak base to form an acidic (pH<7) solution.
- A weak acid reacts with a strong base to form a basic (pH>7) solution.
A suitable indicator should be chosen, that will experience a change in colour close to the end point of the reaction.
Firstly, the burette should be rinsed with the standard solution, the pipette with the unknown solution, and the conical flask with distilled water.
Secondly, a known volume of the unknown concentration solution should be taken with the pipette and placed into the conical flask, along with a small amount of the indicator chosen. The burette should be filled to the top of its scale with the known solution.
The known solution should then be allowed out of the burette, into the conical flask. At this stage we want a rough estimate of the amount of this solution it took to neutralise the unknown solution. Let the solution out of the burette until the indicator changes colour and then record the value on the burette. This is the first titre and should be discluded from any calculations.
Perform three more titrations, this time more accurately, taking into account we know roughly where the end point will occur. Take note of each of the readings on the burette at the end point, and average these at the end. Stop when the lightest shade of pink is visible (and doesn't disappear). This should make sure that there is the right number of moles for the calculations.
Calculations
Knowing the average of the three results recorded we can work out the number of moles, and hence the concentration of the unknown solution.
Firstly, work out and balance the equation of the reactants. It can now be determined how many moles of reactants will neutralize a mole of the solution.
We know the number of moles of the standard solution used (concentration multiplied by volume) and can then use this to determine the moles in the unknown solution. By then dividing this by the volume of the unknown solution, we can work out the concentration.
When making a graph of the titration, two points that will help you in your calculations are the half-way point and the equivalence point. At the half-way point, the pH (-log(H+)) is equal to the pKa/pKb, the -log(Ka/Kb). Using this information, you can determine the Ka or Kb of the acid/base from the graph of the solution. At the equivalence point, the acid and base have neutralized each other to produce water and a salt (in most acid-base reactions). Therefore, since all of the acid/base added has be neutralized, the moles of acid=moles of base. This is helpful in standardizations, when you know the molarity of either the acid or the base, and you're trying to find the molarity of the other solution in the titration. In such cases, knowing the molarity of one solution (moles/Liter), as well as the volume (Liters), you can determine moles; this number of moles is the same for the other solution of unknown molarity. Then, by dividing the moles by the volume of this solution present, you can calculate the molarity!
