Phenolphthalein
|
|
Phenophthalein.png
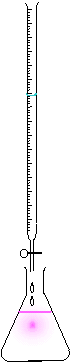
Phenolphthalein is a sensitive pH indicator with the formula C20H14O4. Often used in titrations, it turns from colorless in acidic solutions to pink in basic solutions, the color change occurring between pH 8 and pH 10. If the concentration of indicator is particularly strong, it can appear purple.
In strongly basic solutions, phenolphthalein's pink color undergoes a rather slow fading reaction and becomes colorless again. In other words, the molecule has three forms:
| H2Phenolphthalein acidic no color | ↔ | Phenolphthalein2- basic pink | ↔ | Phenolphthalein(OH)3- strong alkaline no color |
The fading reaction is sometimes used in undergraduate classes for the study of reaction kinetics.
Phenolphthalein is insoluble in water, and is usually dissolved in alcohols for use in experiments. It is itself a weak acid, which can lose H+ ions in solution. The phenolphthalein molecule is colorless. However, the phenolphthalein ion is pink. When a base is added to the phenolphthalein, the atom ↔ ions equilibrium shifts to the ionization because H+ ions are removed, (by Le Chatelier's principle).
Phenolphthalein has been used for over a century as a laxative, but is now being removed from the market because of concerns over carcinogenity. However, the small amounts usually used in experiments are harmless. Phenolphthalein is also commonly used in a mixture, primarily by forensic scientists, to test for the presence of blood.
External links
- Page on different titration indicators, including phenolphthalein (http://www.chemguide.co.uk/physical/acidbaseeqia/indicators.html)
- http://wwwsoc.nii.ac.jp/jsac/analsci/pdfs/a15_0611.pdfde:Phenolphthalein
ja:フェノールフタレイン nl:Fenolftaleïne pt:Fenolftaleína sv:Fenolftalein
