Naphthalene
|
|
Naphthalene (also known as naphthalin, naphthaline, tar camphor, white tar, albocarbon, or naphthene) is a crystalline white solid hydrocarbon with a typical mothball odor. Naphthalene is volatile, forming a flammable vapor. Its molecules consist of two fused benzene rings. It is manufactured from coal tar, and converted to phthalic anhydride for the manufacture of plastics, dyes and solvents. It is also used as an antiseptic and insecticide, especially in mothballs. p-Dichlorobenzene is now often used instead of naphthalene as a mothball substituent.
| Naphthalene | |
|---|---|
| Chemical name | Naphthalene |
| Chemical formula | C10H8 |
| Molecular mass | 128.17 g/mol |
| Melting point | 80.6 °C |
| Boiling point | 218 °C |
| CAS number | 91-20-3 |
| SMILES | C1(C=CC=C2)=C2C=CC=C1 |
| Missing image Naphthalene_chemical_structure.png Chemical structure of naphthalene | |
| Contents |
Name
The name naphthalene is derived from the Latin word of Iranian origin naphtha. Naphtha is a term for any volatile and usually flammable liquid hydrocarbon mixture, normally in the context of a solvent. It was earlier spelled naphthaline. The name phthalic acid is a shortened naphthalic acid, which is named from naphthalene.
Structure and reactivity
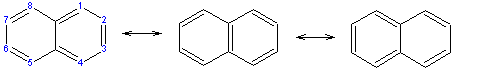
A naphthalene molecule is composed of two fused benzene rings. (In organic chemistry, rings are fused if they share two or more atoms). Accordingly, naphthalene is classified as a benzenoid polycyclic aromatic hydrocarbon (PAH). Naphthalene has three resonance structures, which are shown in the above drawing. Naphthalene has two sets of equivalent hydrogens. The alpha positions are positions 1, 4, 5, and 8 on the above drawing. The beta positions are positions 2, 3, 6, and 7.
Unlike benzene, the carbon-carbon bonds in naphthalene are not of the same length. The bonds C1C2, C3C4, C5C6 and C7C8 are about 1.36Å in length, whereas all the other carbon-carbon bonds are about 1.42Å in length. This has been verified by x-ray diffraction and can be expected from the resonance structures, where the bonds C1C2, C3C4, C5C6 and C7C8 are double in two of the three structures, whereas all the others are double in only one.
Like benzene, naphthalene can undergo electrophilic substitution. The major product generally has the electrophile in the "alpha" position. Sulfonation, however, gives a mixture of the "alpha" product 1-naphthalenesulfonic acid and the "beta" product 2-naphthalenesulfonic acid, with the ratio dependent on reaction conditions. Naphthalene can be hydrogenated under high pressure or with a special catalyst to give decalin (C10H18, also known as bicyclo[4.4.0]decane). Oxidation of naphthalene with chromate or permanganate, or catalytic oxidation with O2 and a vanadium catalyst, gives phthalic acid.
Health effects
In humans, exposure to large amounts of naphthalene may damage or destroy red blood cells. This could cause the body to have too few red blood cells until it replaces the destroyed cells. Humans, particularly children, have developed this condition after ingesting mothballs or deodorant blocks containing naphthalene. Some of the symptoms of this condition are fatigue, lack of appetite, restlessness, and pale skin. Exposure to large amounts of naphthalene may also cause nausea, vomiting, diarrhea, blood in the urine, and a yellow color of the skin.
When the U. S. National Toxicology Program exposed male and female rats and mice to naphthalene vapors on weekdays for two years (1), male and female rats exhibited: clear evidence of carcinogenic activity, based on increased incidences of adenoma and neuroblastoma of the nose, female mice exhibited some evidence of carcinogenic activity, based on increased incidences of alveolar and bronchiolar adenomas of the lung, and male mice exhibited no evidence of carcinogenic activity.
The International Agency for Research on cancer (IARC) (2) classifies naphthalene as possibly carcinogenic to humans [Group 2B]. It also points out that acute exposure causes cataracts in humans, rats, rabbits, and mice and, that haemolytic anaemia, described above, can occur in children and infants after oral or inhalation exposure or after maternal exposure during pregnancy.
External Links
- Naphthalene (PIM 363) (http://www.inchem.org/documents/pims/chemical/pim363.htm) - mostly on toxicity of naphthalene