Friedel-Crafts reaction
|
|
The Friedel-Crafts reactions were developed by Charles Friedel and James Crafts in 1877. There are two main types of Friedel-Crafts reactions: alkylation reactions and acylation reactions.
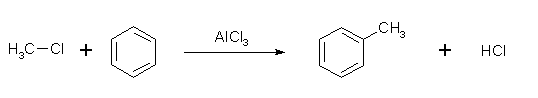
Friedel-Crafts alkylation
Friedel-Crafts alkylation involves a benzene ring and an alkyl chloride. With aluminium chloride as a catalyst, the alkyl group attaches at the former site of the chloride ion.
This reaction has one big disadvantage, namely that the product is more nucleophilic than the reactant due to the electron donating alkyl-chain. Therefore, another hydrogen is substituted with an alkyl-chain, which leads to overalkyation of the molecule. Also, if the chlorine is not on a tertiary carbon, carbocation rearrangement will occur. This is due to the relative stability of the tertiary carbocation over the seondary and primary carbocations.
Friedel-Crafts acylation
Friedel-Crafts acylation involves a benzene ring and an acyl chloride. This reaction has several advantages over alkylation. One is that the product is always less reactive than the original molecule, so multiple acylations do not occur. Also, there is no carbocation rearrangement as an aromatic carbocation is very stable compared to alkyl carbocations. As an added bonus, the ketones produced by these reactions can be reduced using a Clemmensen reduction.
Friedel-Crafts_acylation_of_benzene_by_ethanol_chloride.png
Friedel-Crafts acylation of benzene by ethanoyl chloride
See also: Aluminium chloridede:Friedel-Crafts-Alkylierung fr:Alkylation de Friedel-Crafts pl:Reakcja Friedla-Craftsa