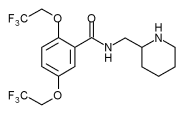
Flecainide
|
|
| N-(2-piperidinylmethyl)-2,5- bis(2,2,2-trifluoroethoxy)benzamide | |
| CAS number 54143-55-4 | ATC code C01BC04 |
| Chemical formula | C17H20F6N2O3 |
| Molecular weight | 474.40 |
| Bioavailability | 95% |
| Metabolism | CYP2D6 (limited) |
| Elimination half life | 20 (range 12-27) hours |
| Excretion | Renal |
| Pregnancy category | C |
| Legal status | ? |
| Delivery | tablets 50, 100, 150 mg |
Flecainide acetate is a class Ic antiarrhythmic agent used to prevent and treat tachyarrhythmias (abnormal fast rhythms of the heart). It is used to treat a variety of cardiac arrhythmias including paroxysmal atrial fibrillation (episodic irregular heartbeat originating in the upper chamber of the heart), paroxysmal supraventricular tachycardia (episodic rapid but regular heartbeat originating in the atrium), and ventricular tachycardia (rapid rhythms of the lower chambers of the heart). Flecainide works by regulating the flow of sodium in the heart, thus slowing nerve impulses.
Flecainide was originally sold under the trade name Tambocor® (manufactured by 3M pharmaceuticals). Flecainide went off-patent on February 10th, 2004, and is now available under the trade names Almarytm®, Apocard®, Ecrinal®, and Flécaine®. It is also available generically.
| Contents |
Uses
Flecainide is used in the treatment of many types of supraventricular tachycardias, including AV nodal reciprocating tachycardia (AVNRT) and Wolff-Parkinson-White syndrome (WPW). This is because of the action of flecainide on the His-Purkinje system.
It also has limited use in the treatment of certain forms of ventricular tachycardia (VT). In particular, flecainide has been useful in the treatment of ventricular tachycardias that are not in the setting of an acute ischemic event. It has use in the treatment of right ventricular outflow tract (RVOT) tachycardia1 and in the suppression of arrhythmias in arrhythmogenic right ventricular dysplasia (ARVD)2. However, studies have shown an increased mortality when flecainide is used to suppress ventricular extrasystoles in the setting of acute myocardial infarction.3,4
In individuals suspected of havings the Brugada syndrome, the administration of flecainide may help reveal the ECG findings that are characteristic of the disease process. This may help make the diagnosis of the disease in equivocal cases.5
Flecainide has been introduced into the treatment of arrhythmias in the pediatric population.
Dosing
The dosing of flecainide is varied, with consideration made to the individual's other medications and comorbid conditions and how they may affect the metabolism of flecainide. Individuals with significant renal impairment may require measurement of the plasma level of flecainide to insure that the drug level remains within the therapeutic range (ie: that toxic levels do not occur). In addition, lower drug levels may be saught for the treatment of benign arrhythmias, to lower the chance of inducing a toxic effect of the drug. When used in the pediatric population, the dose of flecainide may be adjusted to the individual's body surface area.
Given the variable half life of flecainide and the characteristic QT prolongation on ECG elicited in flecainide toxicity, starting flecainide or changing the level of the drug is done under telemetry monitoring (preferably in a hospital telemetry unit) until a steady state plasma level has been achieved, typically three to five days after the dose has been increased.
For the treatment of supraventricular tachycardias and paroxysmal atrial fibrillation or flutter in individuals without significant structural heart disease, a starting dose of 50 mg twice a day may be appropriate. The dose may be increased (once a steady state level has been reached) if breakthrough arrhythmias occur.
For the treatment of life-threatening ventricular arrhythmias (ie: ventricular tachycardia), a starting dose of 100 mg twice a day may be appropriate. As with the treatment of benign arrhythmias, the dose of flecainide given for the treatment of life-threatening ventricular arrhythmias should not be increased until a steady state has been achieved.
Mechanism of action
Flecainide works by blocking the Nav1.5 sodium channel in the heart, causing prolongation of the cardiac action potential.6 This thereby slows conduction of the electrical impulse within the heart. The greatest effect is on the His-Purkinje system and ventricular myocardium. The effect of flecainide on the ventricular myocardium causes decreased contractility of the muscle, which leads to a decrease in the ejection fraction.
The effect of flecainide on the sodium channels of the heart increases as the heart rate increases.7 This is known as use-dependance. This means that flecainide is potentially more useful to break a tachyarrhythmia (because it has increased effect during the fast heart rate) than to prevent a tachyarrhythmia from occurring (because of its lowered effectiveness during slower heart rates).
Metabolism and drug interactions
Flecainide has high bioavailability after an oral dose8, meaning that most of the drug that is ingested will enter the systemic blood stream. Peak serum concentrations can be seen 1 to 6 hours after ingestion of an oral dose. While the plasma half life is about 20 hours, it is quite variable, and can range from 12 to 27 hours9. During oral loading with flecainide, a steady state equilibrium is typically achieved in 3 to 5 days.
The majority of flecainide is eliminated by the kidneys, with the remainder metabolised by the cytochrome P450 IID6 isoenzyme in the liver.10 Therefore, alterations in renal function or urine pH will greatly affect the elimination of flecainide, as more is eliminated by the hepatic route.
Because of the dual elimination routes of flecainide and it's tendency to decrease myocardial contractility11, flecainide interacts with numerous pharmaceuticals and can potentiate the effects of other myocardial depressants and AV node blocking agents. In addition, flecainide can decrease the metabolism or elimination of many (but not all) agents that use the cytochrome P450 enzyme system.
A full list of drug interactions with flecainide can be obtained from the manufacturer. Some important drug interactions with flecainide include:
- Alcohol - may further depress normal heart function.
- Amiodarone - inhibits cytochrome P450 IID6 and may increase flecainide levels
- Cimetidine - increases flecainide levels by 30% and half-life by 10%
- Digoxin - may increase digoxin levels
- Paroxetine - increased effect of both drugs.
- Propafenone - increased effect of both drugs and increased risk of toxicity.
- Quinidine - inhibits cytochrome P450 IID6 and may increase flecainide levels
Serious adverse reactions
While there are no absolute contraindications to the administration of flecainide, the dose may need to be adjusted in certain clinical scenarios. As with all other antiarrhythmic agents, there is a risk of proarrhythmia associated with the use of flecainide. This risk is probably increased when flecainide is co-administered with other class Ic antiarrhythmics, such as encainide. The risk of proarrhythmia may also be increased by hypokalemia.12 The risk of proarrhythmia is not necessarily associated with the length of time an individual is taking flecainide, and cases of late proarrhythmia have been reported.13 Because of the role of both the liver and the kidneys in the elimination of flecainide, the dosing of flecainide may need to be adjusted in individuals who develop either liver failure or renal failure.
Extreme caution should be used in individuals with an acute myocardial infarction (heart attack), because of the increased risk of death in this group.3,4
Because of the negative inotropic effects of flecainide11, it should be used with caution in individuals with depressed ejection fraction, and may worsen congestive heart failure in these individuals.
As with all class I antiarrhythmic agents, Flecainide increases the capture thresholds of pacemakers.14 Therefore, capture thresholds should be remeasured in individuals with pacemakers after the steady-state flecainide dose is changed.
Toxicity
Due to the narrow therapeutic index of flecainide, physicians should be alert for signs of toxicity before life-threatening arrhythmias occur. Whlie the toxic effects of flecainide are closely related to the plasma levels of the drug15, it is infeasible to check the plasma concentration in an individual on a regular basis.
Signs of flecainide toxicity include marked prolongation of the PR interval and widening of the QRS duration on the surface ECG. There may be signs and symptoms attributable to overt heart failure secondary to sudden decreased myocardial contractility.
Treatment
Treatment of flecainide toxicity involves increasing the excretion of flecainide, blocking it's effects in the heart, and (rarely) institution of cardiovascular support to avoid impending lethal arrhythmias. Modalities that have had success include administration of a beta-sympathomimetic agent15, and administration of a sodium load15 (often in the form of hypertonic sodium bicarbonate). Placing the individual on cardiopulmonary bypass support many be necessary in order to temporarily obviate the need for a beating heart and to increase blood flow to the liver16,17.
Long term effects
In the long term, Flecainide seems to be safe in patients with a healthy heart with no signs of Left ventricular hypertrophy, ischemic heart disease or heart failure.
References
1. Gill JS, Mehta D, Ward DE, Camm AJ. Efficacy of flecainide, sotalol, and verapamil in the treatment of right ventricular tachycardia in patients without overt cardiac abnormality. Br Heart J. 1992 Oct;68(4):392-7. (Medline abstract (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1449923))
2. Sakurada H, Hiyoshi Y, Tejima T, Yanase O, Tokuyasu Y, Watanabe K, Motomiya T, Sugiura M, Hiraoka M. Effects of oral flecainide treatment of refractory tachyarrhythmias. Kokyu To Junkan. 1990 May;38(5):471-6. PMID 2115193.
3. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991 Mar 21;324(12):781-8. PMID 1900101.
4. Greenberg HM, Dwyer EM Jr, Hochman JS, Steinberg JS, Echt DS, Peters RW. Interaction of ischaemia and encainide/flecainide treatment: a proposed mechanism for the increased mortality in CAST I. Br Heart J. 1995 Dec;74(6):631-5. PMID 8541168.
5. Gasparini M, Priori SG, Mantica M, Napolitano C, Galimberti P, Ceriotti C, Simonini S. Flecainide test in Brugada syndrome: a reproducible but risky tool. Pacing Clin Electrophysiol. 2003 Jan;26(1 Pt 2):338-41. PMID 12687841.
6. Ramos E, O'leary ME. State-dependent trapping of flecainide in the cardiac sodium channel. J Physiol. 2004 Oct 1;560(Pt 1):37-49. Epub 2004 Jul 22. PMID 15272045.
7. Wang Z, Fermini B, Nattel S. Mechanism of flecainide's rate-dependent actions on action potential duration in canine atrial tissue. J Pharmacol Exp Ther. 1993 Nov;267(2):575-81. PMID 8246130.
8. Smith GH. Flecainide: a new class Ic antidysrhythmic. Drug Intell Clin Pharm. 1985 Oct;19(10):703-7. PMID 3902429.
9. Padrini R, Piovan D, Busa M, al-Bunni M, Maiolino P, Ferrari M. Pharmacodynamic variability of flecainide assessed by QRS changes. Clin Pharmacol Ther. 1993 Jan;53(1):59-64. PMID 8422742.
10. Haefeli WE, Bargetzi MJ, Follath F, Meyer UA. Potent inhibition of cytochrome P450IID6 (debrisoquin 4-hydroxylase) by flecainide in vitro and in vivo. J Cardiovasc Pharmacol. 1990 May;15(5):776-9. PMID 1692938.
11. Santinelli V, Arnese M, Oppo I, Matarazzi C, Maione S, Palma M, Giunta A. Effects of flecainide and propafenone on systolic performance in subjects with normal cardiac function. Chest. 1993 Apr;103(4):1068-73. PMID 8131440.
12. Ohki R, Takahashi M, Mizuno O, Fujikawa H, Mitsuhashi T, Katsuki T, Ikeda U, Shimada K. Torsades de pointes ventricular tachycardia induced by mosapride and flecainide in the presence of hypokalemia. Pacing Clin Electrophysiol. 2001 Jan;24(1):119-21. PMID 11227957.
13. Morganroth J. Early and late proarrhythmia from antiarrhythmic drug therapy. Cardiovasc Drugs Ther. 1992 Feb;6(1):11-4. PMID 1533532.
14. Fornieles-Perez H, Montoya-Garcia M, Levine PA, Sanz O. Documentation of acute rise in ventricular capture thresholds associated with flecainide acetate. Pacing Clin Electrophysiol. 2002 May;25(5):871-2. PMID 12049386.
15. Winkelmann BR, Leinberger H. Life-threatening flecainide toxicity. A pharmacodynamic approach. Ann Intern Med. 1987 Jun;106(6):807-14. PMID 3107447.
16. Corkeron MA, van Heerden PV, Newman SM, Dusci L. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999 Aug;27(4):405-8. PMID 10470398.
17. Yasui RK, Culclasure TF, Kaufman D, Freed CR. Flecainide overdose: is cardiopulmonary support the treatment? Ann Emerg Med. 1997 May;29(5):680-2. PMID 9140253.
External Links
- 3M Pharmaceuticals (http://products3.3m.com/catalog/us/en001/government/innovative_solutions/node_GSYQ1KL450be/root_GS3RBW6QFVgv/vroot_31S2JJ7584ge/gvel_KJ6SPF8WRSgl/theme_us_innovativesolutions_3_0/command_AbcPageHandler/output_html)
- Family Practice Notebook (http://www.fpnotebook.com/CV190.htm)
- Medline Plus (http://www.nlm.nih.gov/medlineplus/druginfo/uspdi/202240.html)
- Atrial Fibrillation Foundation (http://www.affacts.org/Medications/flecainide.html)
- RxList (http://www.rxlist.com/cgi/generic2/flecainide.htm)
- Cleveland Clinic (http://www.clevelandclinic.org/health/health-info/docs/0600/0686.asp?index=4834&src=news)
- Drugs.com (http://www.drugs.com/MTM/flecainide.html)
- WholeHealthMD.com (http://www.wholehealthmd.com/refshelf/drugs_view/1,1524,254,00.html)
- HealthSquare.com (http://www.healthsquare.com/newrx/tam1424.htm)pt:Flecainida