Emission spectrum
|
|
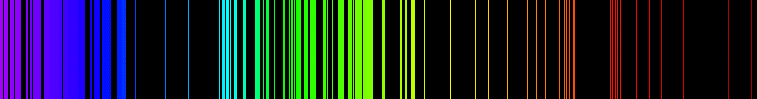
A material's emission spectrum is the amount of electromagnetic radiation of each frequency it emits when it is heated (or more generally when it is excited).
When the electrons in the element are excited, they jump to higher energy levels. As the electrons fall back down, and leave the excited state, energy is re-emitted, the wavelength of which refers to the discrete lines of the emission spectrum. Note however that the emission extends over a range of frequencies, an effect called spectral line broadening.
The term often refers to the visible light emission spectrum, although it extends to the whole electromagnetic spectrum, from the low energy radio waves up to high energy gamma rays.
The emission spectrum can be used to determinate the composition of a material, since it is different for each element of the periodic table. For example, it is used to identify the composition of stars by analysing the received light.
Interestingly, the emission spectrum of an element is the exact opposite of its absorption spectrum; that is, the frequencies emitted by a material when heated are the only frequencies that will be absorbed when it is lighted with a white light.

See also: Rydberg formulahe:ספקטרום פליטה
