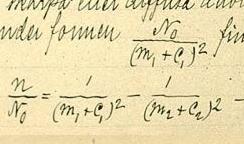Rydberg formula
|
|
The Rydberg formula (Rydberg-Ritz formula) is used in atomic physics for determining the full spectrum of light emission from hydrogen, later extended to be useful with any element.
The spectrum are the wavelengths of photons emitted when electrons jump between discrete energy levels, "shells" around the atom of a certain chemical element.
The fomula was invented by the Swedish physicist Janne Rydberg and presented on November 5, 1888.
Rydberg formula for hydrogen
- <math>\frac{1}{\lambda_{\mathrm{vac}}} = R_{\mathrm{H}} \left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)<math>
Where
- <math>\lambda_{\mathrm{vac}}<math> is the wavelength of the light emitted in vacuum.
- <math>R_{\mathrm{H}}<math> is the Rydberg constant for hydrogen.
- <math>n_1<math> and <math>n_2<math> are integers such that <math>n_1 < n_2<math>.
By setting <math>n_1<math> to 1 and letting <math>n_2<math> run from 2 to infinity, the spectral lines known as the Lyman series converging to 91nm are obtained, in the same manner:
| <math>n_1<math> | <math>n_2<math> | Name | Converge toward |
| 1 | <math>2 \rightarrow \infty<math> | Lyman series | 91nm |
| 2 | <math>3 \rightarrow \infty<math> | Balmer series | 365nm |
| 3 | <math>4 \rightarrow \infty<math> | Paschen series | 821nm |
Rydberg formula for any hydrogen-like element
The formula above can be extended for use with any hydrogen-like chemical elements.
- <math>\frac{1}{\lambda_{\mathrm{vac}}} = RZ^2 \left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)<math>
where
- <math>\lambda_{\mathrm{vac}}<math> is the wavelength of the light emitted in vacuum;
- <math>R<math> is the Rydberg constant for this element;
- <math>Z<math> is the atomic number, i.e. the number of protons in the atomic nucleus of this element;
- <math>n_1<math> and <math>n_2<math> are integers such that <math>n_1 < n_2<math>.
It's important to notice that this formula can be applied only to hydrogen-like, also called hydrogenic atoms chemical elements, i.e. atoms with only one electron on external system of orbitals. Examples would include He+, Li2+, Be3+ etc.de:Rydberg-Formel sl:Rydbergova formula