Salicylic acid
|
|
| Salicylic acid | |
|---|---|
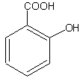
| Chemical name | 2-Hydroxybenzoic acid |
| Chemical formula | C7H6O3 |
| Molecular mass | 138.12 g/mol |
| Melting point | 159 °C |
| Boiling point | 211 °C at 2666 Pa (reduced pressure) |
| CAS number | 69-72-7 |
| SMILES | OC1=CC=CC=C1C(O)=O |
| |
Salicylic acid is a colorless, crystalline organic carboxylic acid. It is usually prepared by Kolbe synthesis (aka Kolbe-Schmitt reaction). Salicylic acid functions as a plant hormone; see Salicylic acid (plant hormone).
Salicylic acid is also found in plants, especially in fruit, in the form of its ester methyl salicylate.
It is toxic if ingested in large quantities, but in small quantities is used as a food preservative and antiseptic in toothpaste. The carboxyl group (–COOH) can react with alcohols, forming several useful esters. The hydroxyl group (–OH) can react with acetic acid to form acetylsalicylic acid. Salicylic acid can also trap oxygen (O2) and initiate free radical reactions.
Salicylic acid is the key additive in many skin-care products for the treatment of acne, keratosis pilaris and warts. It treats acne by causing skin cells to slough off more readily, preventing pores from clogging up.
The medicinal properties of salicylate (mainly the lowering of fever) have been known since ancient times. The substance occurs in the bark of willow trees and the name Salicylic acid is derived from the Latin name for the Willow tree - Salix.
Suppliers/Manufacturers
- Lancaster (http://www.lancastersynthesis.com/homecatsearch.htm) (now part of Alfa)
- Fisher (https://www1.fishersci.com/index.jsp)
- VWR (http://www.vwr.com/index.htm)
- Alfa (http://www.alfa.com/alf/index.htm)
- Aldrich (http://www.sigmaaldrich.com)
- Aurora Fine Chemicals (http://www.aurora-feinchemie.com)
See also
Template:Acne Agentsbg:Салицилова киселина da:salicylsyre de:Salicylsäure fr:Acide salicylique ja:サリチル酸 pl:Kwas acetylosalicylowy
