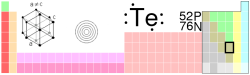
Tellurium
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | tellurium, Te, 52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series | metalloids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 16 (VIA), 5, p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density, Hardness | 6240 kg/m3, 2.25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery lustrous gray
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic weight | 127.60 amu | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 140 (123)pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 135 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| van der Waals radius | 206 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr]4d10 5s2 5p4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| e- 's per energy level | 2, 8, 18, 18, 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states (Oxide) | ?2, 4, 6 (mildly acidic) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Hexagonal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State of matter | Solid (nonmagnetic) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 722.66 K (841.12 ?F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1261 K (1810 ?F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 20.46 ×10-6 m3/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 52.55 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 17.49 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | 23.1 Pa at 272.65 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2610 m/s at 293.15 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.1 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 202 J/(kg*K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical conductivity | 200 /(m·ohm) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 2.35 W/(m*K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1st ionization potential | 869.3 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd ionization potential | 1790 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd ionization potential | 2698 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4th ionization potential | 3610 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5th ionization potential | 5668 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6th ionization potential | 6820 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7th ionization potential | 13200 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tellurium is a chemical element in the periodic table that has the symbol Te and atomic number 52. A brittle silver-white metalloid which looks like tin, tellurium is chemically related to selenium and sulfur. This element is primarily used in alloys and as a semiconductor.
| Contents [hide] |
Notable characteristics
Tellurium is a relatively rare element, in the same chemical family as oxygen, sulfur, selenium, and polonium (the chalcogens).
When crystalline, tellurium is silvery-white and when it is in its pure state it has a metallic luster. This is a brittle and easily pulverized metalloid. Amorphous tellurium is found by precipitating it from a solution of tellurous or telluric acid (Te(OH)6). However, there is some debate whether this form is really amorphous or made of minute crystals. Tellurium is a p-type semiconductor that shows a greater conductivity in certain directions which depends on atomic alignment.
Chemically related to selenium and sulfur, the conductivity of this element increases slightly when exposed to light. It can be doped with copper, gold, silver, tin, or other metals. Tellurium has a greenish-blue flame when burned in normal air and forms tellurium dioxide as a result. When in its molten state, tellurium is corrosive to copper, iron, and stainless steel.
Applications
It is mostly used in alloys with other metals. It is added to lead to improve its strength, durability and to decreases the corrosive action of sulfuric acid. When added to stainless steel and copper it makes these metals more workable. Other uses:
- It is alloyed into cast iron for chill control.
- Used in ceramics.
- It is used in chalcogenide glasses.
- Bismuth telluride (Bi2Te3) has found use in thermoelectric devices.
Tellurium is also used in blasting caps, and has potential applications in cadmium telluride (CdTe) solar panels. Some of the highest efficiencies for solar cell electric power generation have been obtained by using this material, but this application has not yet caused demand to increase significantly. If some of the cadmium in CdTe is replaced by zinc then CdZnTe is formed which is used in solid-state x-ray detectors.
Alloyed with both cadmium and mercury, to form mercury cadmium telluride, an infrared sensitive semiconductor material is formed.
History
Tellurium (Latin tellus meaning "earth") was discovered in 1782 by the Hungarian Franz-Joseph M?von Reichenstein (M?Ferenc) in Transylvania. In 1798 it was named by Martin Heinrich Klaproth who earlier isolated it.
The 1960s brought growth in thermoelectric applications for tellurium, as well as its use in free-machining steel, which became the dominant use.
Occurrence
Tellurium is sometimes found in its native form, but is more often found as the telluride of gold (calaverite), and combined with other metals. The principal source of tellurium is from anode sludges produced during the electrolytic refining of blister copper. It is a component of dusts from blast furnace refining of lead.
Commercial-grade tellurium, which is not toxic, is usually marketed as minus 200-mesh powder but is also available as slabs, ingots, sticks, or lumps. The yearend price for tellurium in 2000 was US$ 14 per pound.
See also: Telluride, Colorado
Compounds
Tellurium is in the same series as sulfur and selenium and forms similar compounds. A compound with metal or hydrogen and similar ions is called a telluride. Gold and silver tellurides are considered good ore.
Isotopes
There are 30 known isotopes of tellurium with atomic masses that range from 108 to 137. Naturally found tellurium consists of eight isotopes (listed in the table to the right) .
Precautions
Humans exposed to as little as 0.01 mg/m3 or less in air develop "tellurium breath", which has a garlic-like odor. Tellurium and tellurium compounds should be considered to be toxic and need to be handled with care. Template:Chem clipart
References
- Los Alamos National Laboratory – Tellurium (http://periodic.lanl.gov/elements/52.html)