Copper
|
|
| |||||||||||||||||||
| General | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
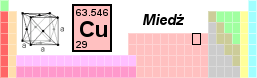
| Name, Symbol, Number | copper, Cu, 29 | ||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||
| Group, Period, Block | 11 , 4, d | ||||||||||||||||||
| Density, Hardness | 8920 kg/m3, 3.0 | ||||||||||||||||||
| Appearance | copper, metallic
| ||||||||||||||||||
| Atomic properties | |||||||||||||||||||
| Atomic weight | 63.546 amu | ||||||||||||||||||
| Atomic radius (calc.) | 135 (145) pm | ||||||||||||||||||
| Covalent radius | 138 pm | ||||||||||||||||||
| van der Waals radius | 140 pm | ||||||||||||||||||
| Electron configuration | [Ar]3d104s1 | ||||||||||||||||||
| e- 's per energy level | 2, 8, 18, 1 | ||||||||||||||||||
| Oxidation states (Oxide) | 2,1 (mildly basic) | ||||||||||||||||||
| Crystal structure | cubic, face-centered | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| State of matter | solid (diamagnetic) | ||||||||||||||||||
| Melting point | 1357.6 K (1984.3 ?F) | ||||||||||||||||||
| Boiling point | 2840 K (4653 ?F) | ||||||||||||||||||
| Molar volume | 7.11 ×10-6 m3/mol | ||||||||||||||||||
| Heat of vaporization | 300.3 kJ/mol | ||||||||||||||||||
| Heat of fusion | 13.05 kJ/mol | ||||||||||||||||||
| Vapor pressure | 0.0505 Pa at 1358 K | ||||||||||||||||||
| Speed of sound | 3570 m/s at 293.15 K | ||||||||||||||||||
| Miscellaneous | |||||||||||||||||||
| Electronegativity | 1.9 (Pauling scale) | ||||||||||||||||||
| Specific heat capacity | 380 J/(kg·K) | ||||||||||||||||||
| Electrical conductivity | 59.6 106/(m·Ω) | ||||||||||||||||||
| Thermal conductivity | 401 W/(m·K) | ||||||||||||||||||
| 1st ionization potential | 745.5 kJ/mol | ||||||||||||||||||
| 2nd ionization potential | 1957.9 kJ/mol | ||||||||||||||||||
| 3rd ionization potential | 3555 kJ/mol | ||||||||||||||||||
| 4th ionization potential | 5536 kJ/mol | ||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||
| |||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||
Copper is a chemical element in the periodic table that has the symbol Cu and atomic number 29.
| Contents |
History
In Greek times, the metal was known by the name chalkos (χαλκός). In Roman times, it became known as aes Cyprium (aes being the generic Latin term for copper alloys such as bronze and other metals, and because so much of it was mined in Cyprus). From this, the phrase was simplified to cuprum and then eventually Anglicized into the English copper.
Copper was known to some of the oldest civilizations on record, and has a history of use that is at least 10,000 years old. A copper pendant was found in what is now northern Iraq that dates to 8700 BC. By 5000 BC there are signs of copper smelting, the refining of copper from simple copper oxides such as malachite or azurite. The earliest signs of gold use, by contrast, appear around 4000 BC.
There are copper and bronze artifacts from Sumerian cities that date to 3000 BC, and Egyptian artifacts in copper and copper alloyed with tin nearly as old. In one pyramid, a copper plumbing system was found that is 5000 years old.
Copper_Ingot_Crete.jpg
The Egyptians found that adding a small amount of tin made the metal easier to cast, so bronze alloys are found in Egypt almost as soon as copper is found. Use of copper in ancient China dates to at least 2000 BC. By 1200 BC excellent bronzes were being made in China. Note that these dates are affected by wars and conquest, as copper is easily melted down and reused. In Europe, Oetzi the Iceman, a well preserved male dated to 3200 BC, was found with a copper tipped axe whose metal was 99.7% pure. High levels of arsenic in his hair suggests he was involved in copper smelting.
The use of bronze was so pervasive in a certain era of civilization that it has been named the Bronze Age. The transitional period in certain regions between the preceding Neolithic period and the Bronze Age is termed the Chalcolithic, with some high purity copper tools being used alongside stone tools.
Brass, an alloy of zinc and copper, was known to the Greeks but first used extensively by the Romans.
Copper-symbol.png
Alchemical symbol for copper
Copper was associated with the goddess Aphrodite/Venus in mythology and alchemy, owing to its lustrous beauty, its ancient use in producing mirrors, and its association with Cyprus, which was sacred to the goddess. In alchemy, the symbol for copper was also the symbol for the planet Venus.
Biological role
Copper is essential in all higher plants and animals. Copper is carried mostly in the bloodstream on a plasma protein called ceruloplasmin. When copper is first absorbed in the gut it is transported to the liver bound to albumin. Copper is found in a variety of enzymes, including the copper centers of cytochrome c oxidase, the Cu-Zn containing enzyme superoxide dismutase, and is the central metal in the oxygen carrying pigment hemocyanin. The blood of the horseshoe crab, Limulus polyphemus uses copper rather than iron for oxygen transport.
It is believed that zinc and copper compete for absorption in the digestive tract so that a diet that is excessive in one of these minerals may result in a deficiency in the other. The RDA for copper in normal healthy adults is 0.9 mg/day.
Toxicity
All copper compounds, unless otherwise known, should be treated as if they were toxic. 30g of copper sulfate is potentially lethal in humans. The suggested safe level of copper in drinking water for humans varies depending on the source, but tends to be pegged at 1.5 to 2 mg/L. The DRI Tolerable Upper Intake Level for adults of dietary copper from all sources is 10 mg/day.
An inherited condition called Wilson's disease causes the body to retain copper, since it is not excreted by the liver into the bile. This disease, if untreated, can lead to brain and liver damage. In addition, studies have found that people with mental illnesses such as schizophrenia had heightened levels of copper in their systems. However it is unknown at this stage whether the copper contributes to the mental illness, whether the body attempts to store more copper in response to the illness, or whether the high levels of copper are the result of the mental illness.
Miscellaneous hazards
The metal, when powdered, is a fire hazard. At concentrations higher than 1 mg/L, copper can stain clothes and items washed in water.
Physical characteristics
Copper is a reddish-coloured metal, with a high electrical and thermal conductivity (among pure metals at room temperature, only silver has a higher electrical conductivity). Copper may well be the oldest metal in use, as copper artifacts dating to 8700 BC have been found. Besides being part of various ores, copper can be found in the metallic form (i.e. native copper) in some locations.
There are two stable isotopes, 63Cu and 65Cu, along with a couple dozen radioisotopes. The vast majority of radioisotopes have half lives on the order of minutes or less, the longest lived, 64Cu, has a half life of 12.7 hours, with two decay modes, leading to two separate products.
There are numerous alloys of copper - speculum metal is a copper/tin alloy, brass is a copper/zinc alloy, and bronze is a copper/tin alloy.
Compounds
CopperMineralUSGOV.jpg
Common oxidation states of copper include the less stable copper(I) state, Cu+1; and the more stable copper(II) state, Cu+2, which forms lovely blue or blue-green salts. Under unusual conditions, a +3 state can be obtained.
Copper(II) carbonate is green from which arises the unique appearance of copper-clad roofs or domes on some buildings. Copper(II) sulfate forms a blue crystalline pentahydrate which is perhaps the most familiar copper compound in the laboratory. It is used as a fungicide, known as Bordeau mixture.
There are two stable copper oxides, copper(II) oxide (CuO) and copper(I) oxide (Cu2O). Copper oxides are used to make yttrium barium copper oxide (YBa2Cu3O7-δ) or YBCO which forms the basis of many unconventional superconductors.
Other compounds : Copper(I) chloride, copper(II) chloride, copper(II) sulfide.
Occurrence
Chino_copper_mine.jpg
- See Copper extraction for the main article.
Copper can be found as native copper in mineral form. Minerals such as the carbonates azurite (2CuCO3Cu(OH)2) and malachite (CuCO3Cu(OH)2) are sources of copper, as are sulfides such as chalcopyrite (CuFeS2), bornite (Cu5FeS4), covellite (CuS), chalcocite (Cu2S) and oxides like cuprite (Cu2O).
Most copper extraction happens in large open pit mines in deposits that contain less than one percent copper. Examples include: Chuquicamata in Chile and El Chino mine in New Mexico.
The Intergovernmental Council of Copper Exporting Countries (CIPEC), defunct since 1992, once tried to play a similar role for copper as OPEC does for oil, but never achieved the same influence, not least because the second-largest producer, the United States, was never a member. Formed in 1967, its principal members were Chile, Peru, Zaire, and Zambia.
Applications
Copper is malleable and ductile, and is used extensively, in products such as:
- Copper wire.
- Copper plumbing.
- Doorknobs and other fixtures in houses.
- Statuary: The Statue of Liberty, for example, contains 179,200 pounds (81.3 Mg) of copper.
- Electromagnets.
- Motors, esp electromagnetic motors.
- Watt's steam engine.
- Electrical relays, electrical busbars and electrical switches.
- Vacuum tubes, cathode ray tubes, and the magnetrons in microwave ovens.
- Wave guides for microwave radiation.
- There is increasing use of copper in integrated circuits, replacing aluminium because of its superior conductivity.
- As a component of coins.
- In cookware, such as frying pans.
- Most flatware (knives, forks, spoons) contains some copper (nickel silver).
- Sterling silver, if it is to be used in dinnerware, must contain a few percent copper.
- As a component in ceramic glazes, and to color glass.
- Musical instruments, especially brass instruments.
- As a biostatic surface in hospitals, and to line parts of ships to protect against barnacles and mussels.
- Compounds, such as Fehling's solution, have applications in chemistry.
- Copper (II) sulfate is used as a poison and a water purifier.
References
- Los Alamos National Laboratory - Copper (http://periodic.lanl.gov/elements/29.html)
- Copper: Technology & Competitiveness (Summary) Chapter 6: Copper Production Technology; Author: Office of Technology Assessment 2005