Talk:Carbon dioxide
|
|
| Contents |
Paleo history of CO2
The page currently gives this source for a graph showing CO2 in the distant past: 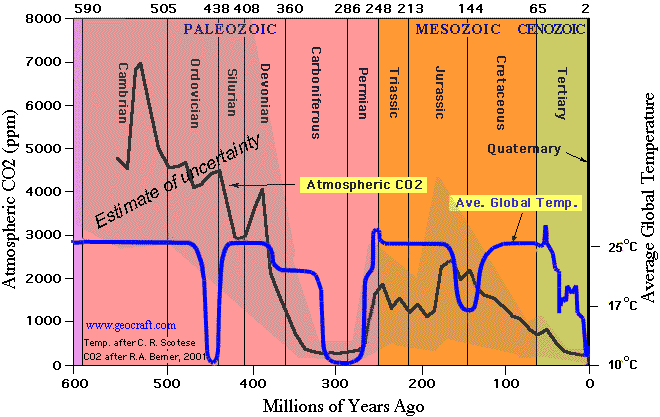 It's schematic, and the temp curve is substantially incorrect. I suggest changing it to Royer et al (2004). http://www.gsajournals.org/gsaonline/?request=get-document&doi=10.1130%2F1052-5173(2004)014%3C4:CAAPDO%3E2.0.CO%3B, Fig 1. I'll do this if no-one objects :) Tom Rees
It's schematic, and the temp curve is substantially incorrect. I suggest changing it to Royer et al (2004). http://www.gsajournals.org/gsaonline/?request=get-document&doi=10.1130%2F1052-5173(2004)014%3C4:CAAPDO%3E2.0.CO%3B, Fig 1. I'll do this if no-one objects :) Tom Rees
Absolute value which is base of increase?
Atmospheric CO2 has increased about 25 percent since the early 1800s, with an estimated increase of 10 percent since 1958
- Anyone know what it increased from? Like, from 0.04% to 0.05% or something? Ed Poor
- Well, everything except nitrogen, oxygen, and argon totals under 1 percent, and is generally measured in parts per million. Those concentrations are significant. Vicki Rosenzweig, Monday, June 24, 2002
- Well, I'm getting better at this Internet-thingie. I just found the answer to my own question!
- "Carbon dioxide, CO2, is one of the gases in our atmosphere, being uniformly distributed over the earth's surface at a concentration of about 0.033% or 330 ppm." [1] (http://scifun.chem.wisc.edu/chemweek/CO2/CO2.html)
Anyone know the ammount of CO2 that nature produces each year? Animals and humans? How much does it consume? 144.173.6.79
- (SEWilco 08:30, 12 Aug 2003 (UTC)) See carbon cycle - production is "source", consumption is "sink".
Density of CO2
(don't quite know how this works, trying my way here...)
I was looking for the density of CO2 at normal conditions, and the best the CO2 page could say was 1.5 times as dense as air. Someone ought to put in a proper number for it (my guess is about 1 g/m3)
- Heikki (at) lsd.dk
- It currently says "Its density at 298K is 1.98 kg m-3, about 1.5 times that of air.", so I think this issue is resolved. SEWilco 19:42, 5 Sep 2003 (UTC)
Significance of CO2 greenhouse, Kyoto
Changed user:SEWilco's "the strongest of the minor greenhouse gases" back to "a greenhouse gas" since "minor greenhouse gas" is not a technical term that conveys any meaning; the only reason for the edit seems to be to attach the word "minor" to CO2's greenhouse activity in order to downplay the global warming connection.
Also corrected the goal of Kyoto: reduction of CO2 emissions, not reduction of CO2 emissions of industrialized countries.
Also added a sentence about debate of size of CO2's effect. AxelBoldt 09:26, 2 Sep 2003 (UTC)
- Read the Kyoto Protocol. It limits the CO2 emissions of industrialized countries, not other countries. It also repeats that the industrialized countries commit themselves to pay all the expenses for developing countries to follow the requirements and deal with climate change (even natural change). The economic issues are repeated several times in the Protocol and the UNFCCC parent document. SEWilco 19:50, 5 Sep 2003 (UTC)
NPOV Global Warming
Ed, please stop this watering down of global warming stuff. Already we have the pointless "controvery" and "hypothesis" articles. Now we have yet another "some". "Some" believe that CO2 is released from the gills of magical leprechauns. -- Tarquin 14:51, 4 Sep 2003 (UTC)
- How can I satisfy you, Tarquin? Is the official position of Wikipedia that CO2 causes global warming? Or is Wikipedia neutral (see NPOV) on this point? --Uncle Ed 15:34, 4 Sep 2003 (UTC)
- as they stand, the stuff on global warming does not seem like NPOV to me. There is far too much emphasis on the objections -- Tarquin 19:49, 4 Sep 2003 (UTC)
- Okay, then let's work together to add more argumens in favor of the global warming theory. Is there any evidence proving CO2 causes global warming, that isn't in the 'pedia but should be? Are there any prominent scientists who endorse the GW view? How about more endorsements from politicians or environmentalist groups (just kidding :-)? Seriously, I'm not looking to make the Wikipedia come out against the GW theory; if there's a lack of balance, let's work together on it. --Uncle Ed 15:10, 5 Sep 2003 (UTC)
- As has been mentioned, there are other pages dealing with greenhouse effect, global warming, global warming/temp (new GW page), climate change, as well as individual components and issues. Can we compartmentalize this so there is only a small summary of CO2's role here, with links for detail? We can't list everything about CO2 here anyway -- its production during combustion should be in an article about the chemistry of combustion, the hazards of CO2 clouds should be mentioned in a volcano article and perhaps a Hazardous Materials data sheet, etc. SEWilco 20:09, 5 Sep 2003 (UTC)
- One thing which should be here is mention of the estimated effect of CO2 in the greenhouse effect, so other articles don't have to mention various other figures. We're doing that in the water vapor article. These will be estimated effects, because we don't understand all the factors yet (yes, the IPCC says that too). But people curious about one material will know where to find the info. Wiki should have facts, but if the facts are fuzzy then that's what should be reported. If we have estimates, listing them will at least help readers see the known range. SEWilco 20:09, 5 Sep 2003 (UTC)
- Oh, I forgot anthropogenic global warming article exists. As "global warming/temp" and "climate change" indicate, there is a difference between the fact that change exists, a temperature change upward ("global warming"), and the cause of climate change (no matter what type of change). SEWilco 20:15, 5 Sep 2003 (UTC)
- Ed, unlike you, I do not think that NPOV is achieved by a list of all arguments. That makes a tennis match, not an encyclopedia article. Just take a look at free trade: it's an unreadable mess. Here's a question for you: which countries do the opponents of GW come from? -- Tarquin 09:04, 6 Sep 2003 (UTC)
- You might be oversimplifying my "thoughts". I don't really believe that listing all arguments is required for neutrality. It is, however, a judgment call to decide whether a particular argument merits inclusion. But to answer your question with a question, as we Jews are noted for doing: which countries do the opponents of "Zionism is racism" come from? --Uncle Ed 14:00, 8 Sep 2003 (UTC)
It's deadly!
- Air with 5% CO2 causes perceptible increased respiration, 6-10% results in shortness of breath, headaches, dizziness, sweating and general restlessness, 10-15% causes impaired coordination and abrupt muscle contractions, 20-30% causes loss of consciousness and convulsions, over 30% can cause death. [2] (http://www.chm.bris.ac.uk/webprojects2003/silvester/Page7CO2.htm)
- Discussion of toxicity must include mention of time periods involved. OSHA limits are 30,000 ppm (3%) for a ten-minute exposure and 5000 ppm (0.5%) for workday exposures. Now, obviously these limits are set within safe margins, but since a prolonged 5% exposure does result in blood acidosis, necessitating "increased respiration", the question is, how long can the body continue to exert itself at that level before exhaustion occurs? I don't know. Shimmin 19:54, 23 Jul 2004 (UTC)
- Update: Here's what the CDC has to say (http://www.cdc.gov/niosh/idlh/124389.html). In summary, NIOSH describes concentrations above 40,000 ppm (4%) as "immediately dangerous to life and health". The basis for this are studies showing intoxication after 30 minutes at 5% and unconcsciousness after a few minutes at 7-10%. Human fatality after 5 minutes at 9% has been documented. Shimmin 22:32, 23 Jul 2004 (UTC)
Deadly springs in Italy or more GWT propaganda?
[3] (http://www.eesi.psu.edu/news/lethal_italian.php) mentions deadly "carbon dioxide springs" in Italy, in the context of unaccounted-for greenhouse gases entering the atmosphere. The researchers give their phone numbers, but don't bother to do the math which shows that by their own estimates, the total from all such "springs" is less than one volcano -- they imply that the springs' contribution rivals that of all the world's volcanoes but admit that no one has measured this contribution. --Uncle Ed 19:49, 23 Jul 2004 (UTC)
CO2 in hemoblobin
This looks like a contradiction to me:
- If the CO2 concentration is too high, then all hemoglobin is saturated with carbon dioxide and no oxygen transport takes place (even if plenty of oxygen is in the air)
- The CO2 bounded to hemoglobin is not competing with oxygen binding since it binds to amino acids rather than hemo molecules.
Is there something I am missing?
--Pinzo 23:39, 4 Sep 2004 (UTC) I found some interesting information about CO2 poisoning (http://yarchive.net/med/co2_poisoning.html). Several mechanisms appear to exist.
- "The CO2 builds up in your blood and acts as a direct anaesthetic. Eventually, you lose consciousness and respiratory drive, and it's a viscious circle from there."
- CO2 makes the pH drop too much.
Maybe CO2 can saturate hemoglobin and block oxygen transport, but it is possible that someone confused CO2 with CO. CO effectively blocks oxygen transport by the hemoglobin. CO2 doesn't (maybe just a little). 194.149.80.4 14:39, 8 Mar 2005 (UTC)
- My understanding (although not one I recall with sufficient confidence to put in the article) is that CO2 does not bind to hemoglobin. However, when there is too much CO2 in the blood, the blood is more acidic, and the cardiovascular system functions less effectively at low pH. Shimmin 18:53, Mar 8, 2005 (UTC)
Plant respiration
I am not sure about this sentence
- Carbon Dioxide is the primary gas respirated by plants
AFAIK plant respirate oxigen, since they are aerobic. CO2 is taken as the raw material for photosynthesis, but they are two separated processes. If there is no counter-argument, I'll change in a few days. --Pinzo 00:03, 5 Sep 2004 (UTC)
Done! --Pinzo 00:03, 5 Sep 2004 (UTC)
- (William M. Connolley 21:57, 12 Sep 2004 (UTC)) Plants have a perfectly normal respiration and exhale CO2 just like almost everything else. It just happens that they photosynthesise too.
Folks - The article says "Plants also emit CO2 during respiration; but on balance they are net sinks of CO2." As a climate scientist, I want to correct the expression "net sink" here.
To be correct, averaged globally and annually, plants are a net sink for CO2 only as long as the global areal coverage of vegetation is INCREASING. And they are a net sink only as long as the area of immature forest is allowed to continue to increase. Once forests reach maturity, they emit (through oxidation of dead plant matter in Fall and Winter) as much CO2 as they take in (during photosynthesis in Spring and Summer).
If the global areal coverage of vegetation is decreasing (as through deforestation not balanced by regrowth) or is at steady state (as when deforestation is exactly balanced by regrowth), plants are NOT a net sink for CO2.
It might help to be clear that climate change scientists try to teach the concept of short term and long term sources and sinks for CO2.
By SHORT term, we mean time intervals on the order of several years or less. LONG term means decades, centuries, millennia and longer.
A SOURCE is any process that results in the addition of CO2 to the atmosphere.
A SINK is any process that removes CO2 from the atmosphere.
The increased rate of photosynthesis during the spring and summer of each hemisphere makes the plants in that hemisphere a SHORT TERM SINK for CO2. The decomposition (oxidation) of organic matter in fall and winter makes those same plants a SHORT TERM SOURCE of CO2.
This cycle is what gives rise to the annual oscillations in the CDIAC observatory measurements of atmospheric CO2 concentrations (see http://cdiac.esd.ornl.gov/trends/co2/sio-mlo.htm for the Mauna Loa Observatory data, for example).
If you are an educator and would like sggestions for use of this information in science, applied math and social studies classrooms (Grades 8-12), go to http://www.whoi.edu/science/GG/people/kbice/k-12_resources.html. You can also find there some discussion of the long term sources and sinks for CO2, but that discussion is not yet as well developed (as of 2/14/05).
Karen L. Bice, PhD
Percent increase confusion
JonGwynne, go read Percent#Confusion from the use of percentages.
Then come back, and we can discuss redundancy as well. — Gene Nygaard 20:54, 28 Jan 2005 (UTC)
Why not ppm?
In posting a revision, Shimmin asks, "Why artificially add units to an inherently unitless quantity?"
Let me give you just a few of the reasons. First of all, Shimmin, go page back through the history and find the last version in which parts per million were used. Do you see the difference between the symbol used there, and the symbol you used in your revision?
We should use units with dimensionless quantities like this, because "ppm" are inherently ambiguous (and "ppb", etc. are even worse, with long scale vs. short scale considerations, but people who use ppm are likely to use ppb as well).
Parts per million might mean, among other things
- mg/kg
- µL/L
- µm/m
- µmol/mol (and what is being measured still needs to be specified in this case, atoms, molecules, etc.)
- it is often used loosely with liquids for g/m³
- it is used even more loosely with gases for mg/m³
- who knows what else?
- Yes, but ppm, by volume, is not ambiguous. And don't play the SI superiority card and then attempt to use units like µL/L. If it's to be SI, then cm3/m3 are the units for you! If ppm are good enough for peer-reviewed atmospheric science publications, they're good enough for us. Shimmin 05:34, Jan 29, 2005 (UTC)
- It's ambiguous if you don't include in every measurement--and you didn't, unlike some earlier versions of this page. And it still suffers the likelihood of being lost in transmission even if you do. Besides, liters are specifically included as being units acceptable for use with SI--the permanent category for them, not the ones temporarily accepted, so your argument there doesn't hold water either. Not only that, but neither my observations about ppm, nor the reasoning expressed by NIST in this and the related sections about including information with symbols for units and the like, are in any way specific to SI. It doesn't make any differentce if you are talking about something measured in cubic feet per million cubic feet. Or take another one that is used in relation to thermal expansion, where µin/in will identify it as linear expansion rather than volumentric expansion; in any case, ppm is still a stupid thing to use.
- What happens if somebody quotes this sentence from the current page, saying Wikipedia says that "As of 2004, the atmospheric CO2 concentration has increased significantly (approximately 110 ppm) since the start of the Industrial Revolution, with an increase of from 316 ppm since 1959 to 376 ppm in 2003, based on measurements taken at Mauna Loa for an overall increase of 60 ppm over the 44 year history of the Mauna Loa facility."? That information would be lost. Or the whole damn paragraph, for that matter, the information is still lost.
- But that information is not lost if they quote "As of 2004, the atmospheric CO2 concentration has increased significantly (approximately 110 µL/L) since the start of the Industrial Revolution, with an increase of from 316 µL/L since 1959 to 376 µL/L in 2003, based on measurements taken at Mauna Loa or an overall increase of 60 µL/L over the 44 year history of the Mauna Loa facility."
- BTW, that "peer review" applies to the substance of the articles, not the editorial decisions of the editors. They rarely, if ever, review each others work. Gene Nygaard 06:34, 29 Jan 2005 (UTC)
- If ppm is ambiguous, then we should not covert it, because we do not know what it meant originally, and should only quote our original sources. If ppm is unambiguous, then there is not a problem. Shimmin 13:12, Jan 29, 2005 (UTC)
- If you'd follow the link embedded in the text here, you'd see that the editors there (or maybe even the authors themselves) were not foolish enough to allow ppm without identification every time it was used. But what I'm saying is that even when that identification is used, it is often lost in transmission, becoming unidentified "ppm" again the next time someone uses these numbers (and, in writing an encyclopedia, we should expect people to use snippets of information from it). We'd just seen an example of you doing that. Better to use unambiguous units, much less likely to be lost in transmission.
- Even that insistence on identification by either the authors or editors is somewhat surprising, when those editors were foolish enough to let authors who describe each individual measurement as being at best accurate to "0.5 ppmv", yet they play along with them on the notion that from this they can get an annual average with five significant digits accurate to 0.01 ppmv even when those annual averages include missing data filled in by curve fitting other data, etc. I distrust the work of anybody who has no more respect for numbers than that. Gene Nygaard 13:53, 29 Jan 2005 (UTC)
Sure, we can to some extent disambiguate these meanings. The problem is, however, that no matter how carefully that is done in any one publication, when the information is passed on that identifying information very often gets dropped. That is less likely to happen if we use units for this dimensionless quantity.
NIST, in its Guide for the Use of the International System of Units (SI) (http://physics.nist.gov/Pubs/SP811/sp811.html), tells us:
7.10.3 ppm, ppb, and ppt
In keeping with Ref. [6: ISO 31-0], this Guide takes the position that the language-dependent terms part per million, part per billion, and part per trillion, and their respective abbreviations "ppm," "ppb," and "ppt" (and similar terms and abbreviations), are not acceptable for use with the SI to express the values of quantities. Forms such as those given in the following examples should be used instead.
Examples: a stability of 0.5 (µA/A)/min but not: a stability of 0.5 ppm/min
a shift of 1.1 nm/m but not: a shift of 1.1 ppb
a frequency change of 0.35×10-9 f but not: a frequency change of 0.35 ppb
a sensitivity of 2 ng/kg but not: a sensitivity of 2 ppt
the relative expanded uncertainty of the resistance R is Ur = 3 µΩ/Ω
or
the expanded uncertainty of the resistance R is U = 3 × 10-6 R
or
the relative expanded uncertainty of the resistance R is Ur = 3 × 10-6
but not:
the relative expanded uncertainty of the resistance R is Ur = 3 ppm
Because the names of numbers 109 and larger are not uniform worldwide, it is best that they be avoided entirely (in most countries, 1 billion = 1 × 1012, not 1 × 109 as in the United States); the preferred way of expressing large numbers is to use powers of 10. This ambiguity in the names of numbers is one of the reasons why the use of ppm, ppb, ppt, and the like is deprecated. Another, and a more important one, is that it is inappropriate to use abbreviations that are language dependent together with internationally recognized signs and symbols, such as MPa, ln, 1013, and %, to express the values of quantities and in equations or other mathematical expressions (see also Sec. 7.6).
- Note:This Guide recognizes that in certain cases the use of ppm, ppb, and the like may be required by a law or a regulation. Under these circumstances, Sec. 2.1 and Sec. 2.1.1 apply.
(end of quote) Gene Nygaard 00:14, 29 Jan 2005 (UTC)
(William M. Connolley 20:22, 30 Jan 2005 (UTC)) Hmm, hoping not to restart an edit war: I'm very used to seeing ppmv for CO2, and its the standard IPCC usage. However, this is clearly an article about a chemical. So in the atmos section I've added an "or ppm" and linked that to the wiki article on ppm, but only once.
- Like you said, you see "ppmv", and the piped link should reflect that. This "ppmv" is discussed in that article. Gene Nygaard 20:35, 30 Jan 2005 (UTC)
- (William M. Connolley 21:49, 30 Jan 2005 (UTC)) Yes it should be ppmv. But... the article says: As of 2004, the earth's atmosphere is about 0.038%, or 380 µL/L or ppmv, CO2 by volume. so the "v" is slightly awkwardly replicated at the end of the sentence (as it needs to be for µL/L).
- The "by volume should be moved to follow the % figure. I'll do that. Gene Nygaard 22:27, 30 Jan 2005 (UTC)
Percentages
The problem with simply saying that CO2 has increased by 40% is that without context, someone who didn't know any better might falsely be led to believe that this represents an enormous increase in the overall amount of CO2. It is important to put the 110ppm increase in perspective. While the difference between 270ppvm and 380ppvm maybe seem large, that is only because the amounts themselves are so small. Put in perspective, the increase represents 0.01% of the Earth's atmosphere. Maybe you have a better way of expressing this than I have been able to come up with. If so, let's talk about it. But I will absolutely not let the 40% figure stand on its own because it is deceptive. So, as I said before, if we're going to use percentages to illustrate the increase, then we're going to give an accurate picture. Otherwise, we can simply let the raw data stand on its own and talk about increases in terms of ppvm or µL/L or whatever unit of measurement seems to work best. What do you think?--JonGwynne 14:46, 5 Feb 2005 (UTC)
- (William M. Connolley 17:09, 5 Feb 2005 (UTC)) All you're doing is std POV - trying to minimise the effects of CO2.
- I'm not trying to minimize anything. I'm simply putting the thing in perspective. The fact is that the increase of carbon dioxide in the atmosphere is very small. This seems to be a fact you'd like to wave away or disguise with misleading percentages, but if there is evidence that such a small increase of CO2 is going to have such a large effect on climate, why not show evidence of this?--JonGwynne 10:54, 6 Feb 2005 (UTC)
- (William M. Connolley 11:40, 6 Feb 2005 (UTC)) The evidence for the effects on climate is presented elsewhere, of course - it doesn't belong on the CO2 page.
Figures don't lie; but liars figure
Will that bull-headed JonGwynne ever get it through his head that if it is not 1.0001 times some known or knowable, relevant volume, then it is not a "0.01% by volume increase"? See:
- http://www.purplemath.com/modules/percntof.htm an elementary level lesson on percent
- http://www.learningwave.com/lwonline/percent/increase_de.html Percent of Increase or Decrease
- http://faculty.ed.umuc.edu/~swalsh/Math%20Articles/Increase%20Decrease.html How to do Percent Increase and Decrease Problems
- percent#Confusion from the use of percentages
Need more, Jon? Gene Nygaard 14:06, 6 Feb 2005 (UTC)
- Here's some help for an apparent newcomer, I suggest you check out [[4] (http://en.wikipedia.org/wiki/Wikipedia:Civility)] and then consider whether calling someone a liar and "bull-headed" is consistent with the policy.
Then, if you're finished being rude and annoying, maybe you'll stop to consider how best to contribute to resolving this issue. If you have a better way to put the 110ppvm increase in perspective, perhaps you'll share it. It would be a more positive contribution than what you've offered so far. --JonGwynne 15:32, 6 Feb 2005 (UTC)
- If the shoe fits, wear it. See John Peter Zenger. Gene Nygaard 17:25, 6 Feb 2005 (UTC)
- Okay, I'll be constructive and propose a resolution to the conflict. Let's say my water has an arsenic concentration of 10 µg/L, or 0.010 ppm w/v. Now I increase it by a mere 0.01% w/v (User:JonGwynne math, of course), to 100010 µg/L. Then you drink a liter of it. Problem solved. Gene Nygaard 17:58, 6 Feb 2005 (UTC)
- No thanks, I don't think I'll wear your shoes but let's see if I understand your main point. Your "constructive solution" is inviting me to drink poison and die? (70,000 mcg is a acutely lethal dose for humans) (http://www.dartmouth.edu/~toxmetal/TXQAas.shtml). Perhaps you'll explain how suborning another person's death is constructive. It would be amusing to watch you try to rationalize this one. Incidentally, you may have just committed an criminal act but I'll leave it to the system admins to decide whether you should be banned from wikipedia. In the meantime, while wikipedia decorum forbids me to express myself regarding your previous statement, I'll simply direct you to this link (http://www.amishrakefight.org/gfy/) (Note: anyone other than GN who visits the link should keep in mind that it is directed at him rather than them).--JonGwynne 19:27, 6 Feb 2005 (UTC)
- If we start out 80% below the EPA limit, then just have a mere "0.01% increase", just what in the world are you getting so all-fired upset about? Will you just answer that question, so we know where you are coming from? Gene Nygaard 04:41, 7 Feb 2005 (UTC)
- To answer your question, I tend to react angrily when people avocate my death. I take it you went to the link then. Good. So we can return to the discussion at hand? How then would you suggest that we express the fact that the increase in atmospheric CO2 amounts to 0.01% of the total volume of the atmosphere? I take it you don't question the fact that 110ppm represents 0.01%?--JonGwynne 16:49, 7 Feb 2005 (UTC)
- Where's your answer to my question? Did you go study any of those links about how to calculate percent increase? Do you know how to do it? Gene Nygaard 17:24, 7 Feb 2005 (UTC)
- If you want to school me in something, you'd better pick another subject. I learned all about percentages in the 1970s. Yes, I understand that an increase from 270ppm to 380ppm represents an increase of 40%. But, as someone who presumes to lecture others about percentages should know, 100ppm represents 0.01%. Do you agree? (hint: it is a true/false question).
- BTW, since even you aren't stupid enough to fall for those lies when I make them wrt arsenic, I have another important question for you. What makes you think you could pull the wool over anybody's eyes, even if we did let you include your misstatements of fact here? Most people in the world (and especially those who will bother to use an encyclopedia) are a whole lot smarter than you are. It's just an unnecessary distraction that might keep them from figuring out what's going on, for a few seconds. Gene Nygaard 17:33, 7 Feb 2005 (UTC)
- I'm not "trying to pull the wool over anyone's eyes", I'm actively preventing other people from doing exactly that. Oh, and since you claim to know exactly how smart I am as well as how smart everyone else in the world is as well, here's an article which I think pretty accurately describes people who make statements like the one you just did [5] (http://en.wikipedia.org/wiki/Asshole). Go away little boy, your nuisance factor has far exceeded your value to this site. --JonGwynne 23:35, 7 Feb 2005 (UTC)
Truth exposed about another comment by JonGwynne when editing: "Expanding Atmospheric section (BTW, according to the IPCC, it is ppvm) - not a reversion"
Google: ppvm site:ipcc.ch 0 hits Google: ppmv site:ipcc.ch 43 hits
Gene Nygaard 17:24, 7 Feb 2005 (UTC)
- Hey, what do you know? You're right about this one. The greenhouse gas section lists it as ppvm but the original IPCC document lists it as ppmv. Funny that no one caught that until now. The original edit dates back to 12, June 2002. Personally, I always thought it looked a little goofy but since I saw WMC using that form (and is there anyone else here more obsessive about the IPCC?) I just assumed that was the correct format. That'll teach me to assume WMC is correct. ;-> --JonGwynne 23:35, 7 Feb 2005 (UTC)
3 revert rule
How many reverts has User:JonGwynne done to this article today. Appears to me that it is more than three by a bit. Where do we report this behavior? Of course JG will say none, as he never uses revert in edit summaries. -Vsmith 23:40, 6 Feb 2005 (UTC)
- I also hardly ever revert someone else's edit, though I may apply similar edits from time to time as the situation requires. I grew weary of WMC's whining about excessive reverts - though he certainly is the last one to complain about other people reverting his stuff. Come to think of it, you're in a pretty poor position to complain yourself. Aren't you worried about appearing hypocritical?--JonGwynne 23:55, 6 Feb 2005 (UTC)
OK - figured it out: Wikipedia:Administrators' noticeboard/3RR and filed report. -Vsmith 00:39, 7 Feb 2005 (UTC)
Page protected
I'm protecting the page. I've reverted because I notice that "Some environmentalists object to attention being drawn to the proportion of the increase; perhaps because they feel it it an attempt to marginalize the increase. They prefer to emphasize the 40% figure, possibly because it is larger and appears to be much more dramatic than the 0.01% figure and also serves to support their claims that anthropogenic carbon dioxide is a serious problem which needs to be urgently addressed. These environmentalists, some of them very extreme in their views, also don't seem to like discussions of the fact that current estimates of the total effect of all atmospheric carbon dioxide (anthropogenic and otherwise) amounts to 4% of the total greenhouse effect." contains at least one weasel word. I'd suggest you blokes nut it out on the talk page. - Ta bu shi da yu 01:39, 8 Feb 2005 (UTC)
The 0.01% issue
OK, you heard the admin... Let's get this sorted. The issue in question is whether or not to mention that the total increase of CO2 over the entirety of the Industrial Revolution is claimed by experts to amount to 110ppmv which is 0.011% of the total volume of the atmosphere. Does anyone challenge the factual basis of this claim? If not, why not mention it?--JonGwynne 12:58, 8 Feb 2005 (UTC)
- Yes, 110 ppm is equal to 0.0110% (note the last zero for precision), but .0110% is .0110 pph (parts per hundred). Why do we need the redundancy. The other lower numbers with all those leading zeros are absurd as well as redundant and totally unneeded, simply adding clutter (presumably to make the numbers seem less significant, and thereby POV). Vsmith 16:44, 8 Feb 2005 (UTC)
- I wouldn't really mind if you wanted to say .01% instead of 0.01% (or .011 or .0110 if you prefer). It doesn't look right to me without the leading zero but if that's what it takes to get you to sign off on it, then I'll learn to live with it.--JonGwynne 17:18, 8 Feb 2005 (UTC)
- No, perhaps I mistated, it isn't so much that first zero as it is all those between the dec. and the first non-zero number. This is usually solved by sci. notation, but either would be redundant here as the ppmv values are perfectly valid and should be understandable by most serious readers. If not they can easily click to a discussion of ppm. Again, the problem is redundancy and misleading slant. Vsmith 17:45, 8 Feb 2005 (UTC)
- You object to necessary decimal places? There are going to be people for whom scientific notation is either confusing, unfamiliar or both - but not traditional percentages. What's wrong with illustrating the point with traditional percentages. It is an accurate way of conveying the information. It may not be your way, but it is no less correct for that.--JonGwynne 18:01, 8 Feb 2005 (UTC)
- In the literature, the concentration of carbon dioxide in the atmosphere is discussed almost exclusively in units of ppmv. In terms of understanding how the numbers presented in the encyclopedia article relate to numbers they may read in other sources, it seems most sensible to use the units readers are most likely to encounter elsewhere. However, it is also a service to our readers to convert those numbers at least once into other terms they may be more familiar with, such as percentages. The initial mention of the atmospheric carbon dioxide concentration is the sensible place to do this, where we express it in ppmv, in µL/L, and as percentage. Past that point, I prefer to stick with a single set of units. Shimmin 19:00, Feb 8, 2005 (UTC)
(William M. Connolley 21:26, 8 Feb 2005 (UTC)) The idea that simply because something is a "fact" it should be mentioned is not sensible. If you doubt this, I suggest reading http://en.wikipedia.org/wiki/Wikipedia:Requests_for_arbitration/Charles_Darwin/Lincoln_dispute. The "fact" is POV: it is your attempt to minimise the increase in CO2, and its pointless. It also amounts to original research, unless you can find a reputable external source for it.
- I'm not attempting to "minimise the increase in CO2". It can't be minimized. It is what it is. I would prefer to simply state the increase in ppm and leave it at that. You're the one who's insisting not bringing percentages into the discussion. The fact that you are so worried about cosmetics suggests that it is you who are trying to maximize it by calling attention to the 40% increase without putting it in the proper context. Yeah, on its own, 40% sounds like a lot. But when you consider that the 40% is only 0.01% of the total volume of the atmosphere, that puts things in context. When you consider that CO2 contributes to roughly 4% of the overall global warming effect and that even if the affect of extra CO2 is linear (many scientists believe it isn't - they believe that the initial increases from 260ppm had more effect that the later increased have had) that 40% increase in CO2 represents, at best, a 1.6% increase in the total greenhouse effect and, in all likelihood, less than that.--JonGwynne 08:40, 9 Feb 2005 (UTC)
- (William M. Connolley 16:30, 9 Feb 2005 (UTC)) CO2 isn't 4% of the GHE - you made that up. As to the rest: you're going in circles. CO2 inc as a % of total atmos mass is a useless number.
- I didn't make the 4% figure up. I used the numbers given on the greenhouse effect page. It says that the atmosphere, as a whole, absorbs 16% of incoming solar radiation. According to the same page, CO2 accounts for "about 26%" of the effect of all greenhouse gasses combined. Multiply 16% by 26% and you get 4.16%. --JonGwynne 00:16, 10 Feb 2005 (UTC)
- The overall CO2 increase as a percentages of the volume of the entire atmosphere is not a "useless number". Quite the contrary, it is a very usefu one because it puts the increase in context. You just don't like that context because it undermines your "The World is Ending!" mantra. A mere 110ppm increase sounds too insignificant, so you have to tart it up with a percentages to make it seem bigger than it is. What no one likes to talk about is the fact that the scientists currently working on researching climate change have a vested professional interest in turning out dire predictions and proof that human activity is causing the problems they claim to have found. They love to shout down scientists who take money from industry but never reflect on the fact that they're part of an "industry" too: the global-warming research industry. If someone proved that the temperature changes that are showing up are either naturally occurring or maybe not even happening at all, all those atmospheric scientists and climate-model math-wizards working on government grants would suddenly have to find work in the private-sector and some of them have the people-skills of a wolverine on PCP. --JonGwynne 00:16, 10 Feb 2005 (UTC)
- Jon, this is a conspiracy theory, but not a good argument. Reading your comments I ask myself what you know about science? Have you ever done research at an university or a similar instituton? Disqualifying all research work in this area is easy but not very convincing, at least it is not better than any other of the conspiracy theories. -- mkrohn 01:24, 10 Feb 2005 (UTC)
(William M. Connolley 10:01, 10 Feb 2005 (UTC)) Notice also that JG has ignored this point I made above: It also amounts to original research, unless you can find a reputable external source for it. I'm not aware of anyone (let alone anyone reputable) manipulating the data to produce these numbers.
OK, so what have we decided? I would like to know so I can unprotect the page. - Ta bu shi da yu 00:55, 11 Feb 2005 (UTC)
- It appears that most of us are in agreement, or nearly so, that the numerical presentation of the current page is in general acceptable (maybe minor quibbles here and there). However, JonGwynne seems to be obstinately determined to impose his correct view of the numbers and doesn't care to listen to the majority. His determination to impose his version was basically the crux of the problem before and I don't think he has changed his mind. Take a look at the similar ongoing battle over numbers at Greenhouse gas. My reading is that the rest of us (5 or 6 involved in the discussion) could easily reach a consensus on how the numbers should be presented. -Vsmith 02:52, 11 Feb 2005 (UTC)
- Oh please. I have repeatedly stated that I have no problem with a simple numeric presentation (i.e. CO2 has risen X ppm between year Y and Z). It is the persistent attempts to present a once-sided and alarmist percentage and refusal to accept any attempts to place the increases in perspective which are causing the problem.--JonGwynne 21:56, 15 Feb 2005 (UTC)
I don't want to confuse this discussion, but this seems like the right place to suggest that what would be most meaningful to the lay public is to quote the rate of increase in CO2 in ppmv per year, not some percent increase over the preindustrial value. And the truly telling point is that the rate of increase is itself increasing through time. That is why we talk about "acceleration" of the CO2 concentration.
Based on the CDIAC Siple Dome ice core data and the Mauna Loa Observatory data, here are the average rates of increase in CO2 (ppmv/yr) for discrete intervals of time since before the start of the Industrial Revolution:
1650-1744 0.01
1764-1854 0.12
1874-1943 0.28
1953-1965 0.63
1966-1976 1.13
1977-1987 1.48
1994-2003 1.80
If you want to do the linear fits yourself, download the data from: http://cdiac.esd.ornl.gov/trends/co2/siple.htm and http://cdiac.esd.ornl.gov/trends/co2/sio-mlo.htm
Karen L. Bice, Associate Scientist Department of Geology and Geophysics Woods Hole Oceanographic Institution Woods Hole, MA 02536
Another suggestion I meant to make: Preindustrial CO2 was about 280 ppmv. In 2003, CO2 was about 380 ppmv. If you keep "Since the start of the Industrial Revolution, the atmospheric CO2 concentration has increased by ... 40%" the article will be obsolute as soon as published because the CO2 concentration in the atmosphere is rising rapidly.
I suggest wording like, "Before the Industrial Revolution, between 1000 and 1700 AD, the atmospheric CO2 concentration as approximately 280 ppmv. As of 2003, the concentration had increased by 35%, to about 380 ppmv."
It is more words, but is also more accurate.
Karen Bice
Unprotect?
(William M. Connolley 13:37, 17 Feb 2005 (UTC)) I suggest its time to unprotect the page and see what happens. The "will of the majority" is fairly obvious, JG has got himself a few 3RR bans, so we can hope there won't be war. If there is, I suppose you could reprotect. Note that this isn't the only page on which this is happening.
- What about to simply say that "Since ..., the total amount of atmospheric CO2 has increased by 40 percent i.e. by 0.011 percent of the volume of the atmosphere"? The fact that many environmentalists don't like facts that don't sound sufficiently scary is a pretty obvious fact - although unfortunately it's not obvious to everyone - but I am not sure whether this observation belongs to the page about carbon dioxide. Does it sound as a reasonable compromise? --Lumidek 07:02, 20 Feb 2005 (UTC)
- (William M. Connolley 10:28, 20 Feb 2005 (UTC)) This is to restart the argument all over again without reading any of the dialogue. The 0.01% number (a) has no useful value (b) isn't used anywhere else, so amounts to original research.
- 0.01% has no value for those who are not interested in science. I understand that certain facts - like the fact that the CO2 concentration in the atmosphere is negligible anyway - are not convenient for enemies of science who prefer political activism - and who prefer scientific fraud over honest but inconvenient results. But you should not assume that the readers of Wikipedia are people like you. The readers of Wikipedia are people who are, unlike you, interested in these questions and they're looking for data; otherwise they would not open it in the first place. The notation "ppm" incidentally is not quite comprehensible to most non-scientists, and it should be expanded. --Lumidek 14:47, 20 Feb 2005 (UTC)
- How do you translate small volumes to "negligible"? The "effects" of many things present in small proportions are not negligible. I suspect an amount of nerve gas comparable to CO2 concentrations would be considered quite large. Drugs present in the human body in much smaller concentrations are considered quite significant. Similarly for the green house effect of CO2.--Silverback 07:10, 27 Feb 2005 (UTC)
- (William M. Connolley 20:10, 20 Feb 2005 (UTC)) The sole virtue, as far as you (or JG) is concerned, is that its a small number. Your failure to answer either of my points - but especially (b) - is rather painfully obvious.
- There is no "virtue" here. Unlike you, we're not trying to "spin" anything. The number is what it is. Just because you don't like its implications, that doesn't mean you should be entitled to censor it.--JonGwynne 21:56, 20 Feb 2005 (UTC)
- (William M. Connolley 22:01, 20 Feb 2005 (UTC)) Notice how JG has, like Lumidek, failed to answer point (a) or point (b).
- I haven't failed to answer, because you didn't ask a question. You made two statements of your own personal opinion and while you're certainly entitled to it, that doesn't change the fact that it is your personal opinion. Thus, of limited relevance to the matter at hand. --JonGwynne 22:46, 20 Feb 2005 (UTC)
- (William M. Connolley 23:06, 20 Feb 2005 (UTC)) Your failure to asnwer is as painfully obvious as Lumideks. Go answer your RFA instead.
- If you ask a question, I'll answer. You haven't asked one, therefore I haven't answered. As I said before, your personal opinions are your own and you're welcome to them. Just don't go mistaking them for fact. Ooops, too late! ;-> --JonGwynne 23:10, 20 Feb 2005 (UTC)
Compromise
We can't leave this page locked forever because of a dispute over one number. The only way to end the argument is for everyone to move toward an acceptable comprimise. Why can't we just include the pre-industrial ppmv, the current ppmv, and the percent of the total atmosphere that the CO2 now represents? "Since the start of the Industrial Revolution, the atmospheric CO2 concentration has increased from about 260 ppmv (0.0260% of total atmosphere) to 376 ppmv (0.0376% of total) - a 40% increase" - I'm not sure I have all the numbers right, but I think it puts all the information in perspective. It's a little wordy, providing information that the reader could easily calculate, but doesn't something like that say everything that we have been debating whether to include? If we just agree on something like this, the article can finally be unlocked. --Keziah 06:32, 2005 Feb 23 (UTC)
- (William M. Connolley 09:49, 24 Feb 2005 (UTC)) Thats essentially what I want too.
- I note that listing the two different percentages allows comparison between them and uses the same units as given earlier when the percentage is given. This follows the lead of the other atmospheric gases, which mention their respective percentage of present atmosphere. There also is a difficulty in merely presenting the difference between the percentages, as that invites errors such as thinking that the addition of 0.01% of the volume would cause 0.01% change in concentration. The volume of the atmosphere would expand and mess up the concentration. I encourage giving both ppmv and percentage. (SEWilco 09:46, 27 Feb 2005 (UTC))
Page unprotection
I would like to know what the proposed text will be before I unprotect this page. This will give me an idea whether an acceptable compromise has been made. Note here: one party may find they don't like the compromise that much. I'm actually fine with this, but some effort must be made at trying to incorporate their POV. If I can have a response that seems reasonable, then I will unlock the page. - Ta bu shi da yu 05:58, 27 Feb 2005 (UTC)
- I would be content with the current version as, I think, would the vast majority of the editors involved. We may discuss the µL/L or ppmv question and some other tweaks - but a consensus could readily be reached. I note the exception would probably be JonGwynne, whose insistance on his version led to the revert war and the page protection. He is currently the subject of arbitration [6] (http://en.wikipedia.org/wiki/Wikipedia:Requests_for_arbitration/JonGwynne/Proposed_decision) for his behavior here and on a variety of other pages. As a result of that arbitration I would hope he would be less inclined to do battle and more inclined to rational discussion. That however, remains to be seen. Continued protection based primarily on the behavior of that one user is, in my view, unwarrented. -Vsmith 16:02, 27 Feb 2005 (UTC)
- (William M. Connolley 16:46, 27 Feb 2005 (UTC)) I'm happy with the current version too. I could also see some compromises. But *please* let those interested work them out on the page. If it erupts into war again, just re-protect it if someone requests it.
- OK, sorry for taking a while to get back to you guys. Unprotecting now. - Ta bu shi da yu 12:44, 2 Mar 2005 (UTC)
- (William M. Connolley 13:20, 2 Mar 2005 (UTC)) Thanks.
spelling :-)
The original article of 29 Jul 01 referred to it as a colorless gas in the body of the article and that spelling has remained. On 17 Dec 02 a table (or infobox) was added with the spelling: colourless gas. Both spellings have coexisted since. Hmm.. do we need to standardize spelling here? Doesn't make much diff. to me, but colorless appears to have priority. Vsmith 16:26, 23 Mar 2005 (UTC)
- (William M. Connolley 20:22, 23 Mar 2005 (UTC)) It was only the Fixed spelling error that I objected to. Otherwise, I'm happy with the std rules: earliest spelling gets priority if no policies apply. So convert it back but with a reasonable edit comment and I'll be happy :-) Note that there has been a minor outbreak of the sulphate wars at climate change but it seems to have died down.
What's the deal with subscript notation?
The two ways to format subscripts (and superscripts) render identically in my browser (Opera 7.54). So what is the "best" format: '''CO{{sub|2}}''' CO2 or '''CO<sub >2</sub >''' CO2 and why? The former requires fewer keystrokes, although I'm more used to the HTML form. Vsmith 19:33, 16 Apr 2005 (UTC)
- The former form {{sub|2}} uses a template and results in identical HTML as <sub>2</sub> being sent to your browser, hence they would never look any different. Templates live in the template namespace and can be accessed by going to Template:template_name, i.e. Template:sub. The advantage of using templates such a {{stub}} is that it will take whatever text is on the page Template:stub and insert it into all pages including the tag: "{{stub}}". You can also pass parameters to templates to customize them. In the Sub example, the "2" is the parameter being passed to the Template.
- Templates can be used in two ways either {{template}} or {{subst:template}}. The subst: in the second form tells the wiki to immediately substitute the entire current text of template into the page (i.e. {{subst:sub|2}} becomes <sub>2</sub> even in the edit window). The form without the subst: causes the text to inserted only when the page is loaded, i.e. wikipedia looks up the current form of template each time the page is viewed. This has the advantage that changing the text at Template:template can immediately change what is presented in all places it occurs.
- The drawback is that looking up {{template}} each time a page is loaded increases server load. For this reason, some people feel that for things like subscripting, which are both pervasive and unlikely to change, that {{sub|2}} should never be used, and only {{subst:sub|2}} or <sub>2</sub> should be used. After all, each of these produces identical code for the browser. I assume this is why Cburnett replaced the templates. As far as I know, there is no hard and fast policy on this. Dragons flight 22:27, Apr 16, 2005 (UTC)
- Thanks! Now will I remember all that? Guess I'd best apply it soon. :-) Vsmith 23:46, 16 Apr 2005 (UTC)
- Yes, that's why I did it. There's no real need to force subscripts and superscripts to use a template other than ease of editting (I've actually posed an enhancement that would make it easier by doing 4^^th^^ or 4\\th\\ for superscript and CO^^^2^^^ or CO//2// for subscript) since the sub and sup HTML tags will probably never change in HTML. Using templates for such only serves to increase server load... Cburnett 00:26, Apr 17, 2005 (UTC)
CO2-only greenhouse
Is it meaningful to discuss the greenhouse effect of a single atmospheric component, as if that could be divorced from the effects of other atmospheric components? If the IR absorption spectra of, say, CO2 and water overlap at all, then the effect of adding more carbon dioxide will depend on how much water vapor is already in the air. And even if they don't overlap, then the amount of outgoing radiation absorbed by one component depends on the spectrum of the outgoing radiation, which depends on the temperature of the radiation source, which depends on the strength of the greenhouse effect of all the other components.
It seems to me that there are multiple plausible ways to deconvolute the effects of a multiple-component greenhouse, and so this statistic is ripe with potential to spin the result one way or another.
- You are right. While, it is not entirely implausible to define the greenhouse effect of one component (say by asking how much the effect would change by removing all of that component and leaving everything else constant), there are a number of concerns with respect to how one defines that number and people having different ideaologies can reasonably quote different values depending on how they choose to approach the problem. Consequently, any single value is pretty much inherently POV. I am going to remove that recent addition. If the author wants to quote a range of values representative of the NPOV spectrum and add a discussion of the various ways the contribution of CO2 is defined, then I wouldn't object to that. Dragons flight 17:22, Apr 29, 2005 (UTC)
- PS. Please sign your statements on talk pages with 4 tildes ~~~~.
