Statin
|
|
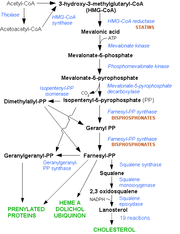
The statins (or HMG-CoA reductase inhibitors) form a class of hypolipidemic agents, used as pharmaceuticals to lower cholesterol levels in people at risk for cardiovascular disease because of hypercholesterolemia.
| Contents |
Members
The statins include (brand names in countries other than the U.S. can differ):
- atorvastatin (Lipitor®)
- fluvastatin (Lescol®)
- lovastatin (Mevacor®, Altocor®, not marketed in the UK)
- pravastatin (Pravachol®, Selektine®, Lipostat®)
- rosuvastatin (Crestor®)
- simvastatin (Zocor®, Lipex®)
- cerivastatin (Lipobay®, Baycol®) - marketing discontinued in 2001 by the manufacturer (Bayer) due to serious side-effects, especially when used in combination with fibrates.
Uses
Statins, the most potent cholesterol-lowering agents, lower LDL-cholesterol (so-called "bad cholesterol") by 30–50%. However, they have less effect than the fibrates in reducing triglycerides and raising HDL-cholesterol ("good cholesterol"). As up to 50% of all circulating cholesterol originates in the diet, doctors in theory only prescribe statins after dietary intervention has not had the desired effect. In practice, diet changes often have little effect, and many doctors omit this step altogether.
The statins play an important role in the primary and secondary prevention of coronary heart disease and myocardial infarction. Research continues into other areas where statins appear to have an effect: inflammation, dementia, and neoplasm (tumors).
Pharmacology
Groups
Two groups of statins exist:
- Fermentation-derived: lovastatin, simvastatin and pravastatin
- Synthetic: fluvastatin, atorvastatin, cerivastatin and rosuvastatin
Fermentation-derived statins appear more effective in reducing LDL, but no clear explanation has accounted for this phenomenon.
Mode of action
Statins act by competitively inhibiting HMG-CoA reductase, an enzyme of the HMG-CoA reductase pathway, the body's metabolic pathway for the synthesis of cholesterol.
Although statins inhibit endogenous cholesterol synthesis, their action goes further than that. By reducing intracellular cholesterol levels, they cause liver cells to upregulate expression of the LDL receptor, leading to increased clearance of Low-Density Lipoprotein from the bloodstream. Dr Michael S. Brown and Dr Joseph L. Goldstein received the Nobel Prize in Physiology or Medicine in 1985 for their work in clarifying this mechanism.
Non-cholesterol related actions
Statins exhibit action beyond lipid-lowering activity in the prevention of atherosclerosis. Doctors believe that statins prevent cardiovascular disease via four proposed mechanisms (all subjects of a large body of biomedical research):
- Improving endothelial function
- Modulate inflammatory responses
- Maintain plaque stability
- Prevent thrombus formation
Despite initial concerns that statins might increase the risk of cancer, various studies have confirmed that statins may reduce cancer risk by up to 50%. A large study (Poynter et al 2005) compared 1953 patients with colorectal cancer and 2015 non-affected controls, and found that people taking statins for over 5 years had reduced their colorectal cancer risk by 50%. Fibrates had no effect. The trialist warn that the number needed to treat would approximate 5000, making statins unlikely tools for primary prevention.
In May 2005, results of an observational study of half a million US veterans showed that people taking statins had a 50% reduced risk of developing any of several types of cancer. One cannot however immediately conclude that the statins had a causal role in reducing cancer risk: patients receiving statins possibly also benefited from unrelated health-related advice. [1] (http://www.newscientist.com/article.ns?id=dn7384)
Safety
While many patients develop muscle cramps and gastrointestinal upset on statin therapy, these usually resolve on temporarily lowering the dose. Liver enzyme derangements may occur, which generally return to normal after briefly discontinuing the drug. Some report headaches. Other side-effects occur rarely.
A main safety concern, rhabdomyolysis (the pathological breakdown of skeletal muscle) may lead to acute renal failure when muscle breakdown products damage the kidney. A 2004 study (Graham et al) found that of 10,000 patients treated for one year, 0.44 will develop this side-effect. All commonly used statins show similar results. (Cerivastatin, which its manufacturer withdrew in 2001, had a much higher incidence - more than 10x higher.) Combining any statin with a fibrate, another category of lipid-lowering drugs, increased the risks for rhabdomyolysis to almost 6.0 per 10,000 person-years.
Most physicians have now abandoned routine monitoring of liver enzymes and creatine kinase, although they still consider this prudent in those on high-dose statins or in those on statin/fibrate combinations, and mandatory in the case of muscle cramps or of deterioration in renal function.
History
Dr Akira Endo and Dr Masao Kuroda of Tokyo, Japan commenced research into inhibitors of HMG-CoA reductase in 1971 (Endo 1992). This team reasoned that certain micro-organisms may produce inhibitors of the enzyme to defend themselves against other organisms, as mevalonate is a precursor of many substances required by organisms for the maintenance of their cell wall (ergosterol) or cytoskeleton (isoprenoids).
The first agent isolated was mevastatin (ML-236B), a molecule produced by Penicillium citrinum. The pharmaceutical company Merck & Co. showed an interest in the Japanese research in 1976, and isolated lovastatin (mevinolin, MK803), the first commercially marketed statin, from the mold Aspergillus terreus.
See also
References
- Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res 1992;33:1569-82. PMID 1464741.
- Furberg CD. Natural statins and stroke risk. Circulation 1999;99:185-188. PMID 9892578.
- Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004;292:2585-90. PMID 15572716.
- Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med 2005;352:2184-92. PMID 15917383.de:Statin