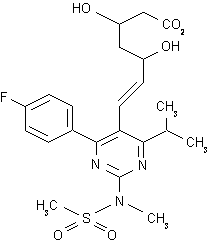
Rosuvastatin
|
|
| Contents |
Presentation
Rosuvastatin is available as Crestor in tablet form (10, 20, or 40 mg) for oral administration. Tablets are pink, round or oval (40 mg), biconvex, film-coated, and imprinted with "ZD4522" and tablet strength.[1] (https://academickids.com:443/encyclopedia/index.php/Rosuvastatin#fn_pill) Japanese approval is in the dose range of 2.5 mg to 20 mg; therefore, smaller dose tablet forms might also be available outside the United States. Note that 97% of worldwide sales have been at or below the 20 mg dose.
Mechanism of action
See the article on statins for more details.
Rosuvastatin is a competitive inhibitor of the enzyme HMG-CoA reductase, having a mechanism of action similar to other statins.
Indications and regulation
Rosuvastatin is indicated for the treatment of elevated LDL cholesterol (dyslipidemia), total cholesterol (hypercholesterolemia) and/or triglycerides (hypertriglyceridemia).[2] (https://academickids.com:443/encyclopedia/index.php/Rosuvastatin#fn_Indications)
As of 2004, rosuvastatin had been approved in 67 countries and launched in 56. Approval in the United States by the FDA came on August 12, 2003.[3] (https://academickids.com:443/encyclopedia/index.php/Rosuvastatin#fn_FDAapproval)
Marketing and competition
Marketing
The drug was billed as a super-statin during its clinical development, claimed to offer a high potency and improved cholesterol reduction compared to rivals in the class. Currently the main competition for Crestor is Vytorin by Merck & Co. (a combination of simvastatin (Zocor) and ezetimibe (Zetia)); unfortunately, there are no published studies showing which of the two drugs is more effective.
First launched in 2003, sales were $129 million and $908 million in 2003 and 2004, respectively, with a total patient treatment population of >4 million by the end of 2004.
Criticisms
Several months after its introduction in Europe, Richard Horton, the editor of the medical journal The Lancet, criticised the way Crestor had been introduced. "AstraZeneca's tactics in marketing its cholesterol-lowering drug, rosuvastatin, raise disturbing questions about how drugs enter clinical practice and what measures exist to protect patients from inadequately investigated medicines," according to his editorial. The Lancet's editorial position is that the data for Crestor’s superiority relies too much on extrapolation from the lipid profile data and too little on hard clinical endpoints, which are available for other statins. The manufacturer responded by claiming that few drugs had been tested so successfully on so many patients. In correspondence published in The Lancet, AstraZeneca's CEO Sir Tom McKillop called the editorial "flawed and incorrect" and slammed the journal for making "such an outrageous critique of a serious, well-studied medicine."[4] (https://academickids.com:443/encyclopedia/index.php/Rosuvastatin#fn_lancetoped)
In 2004, the consumer interest organisation Public Citizen filed a Citzen's Petition with the FDA asking that Crestor be withdrawn from the US market. In 2005, the FDA formally denied this petition.
Some doctors have been hesitant to prescribe rosuvastatin because studies have suggested that this drug has a higher incidence of rhabdomyolysis (a severe undesired side effect) than other statins; this negative impact on sales performance has been much more pronounced in the United States than in other countries. The FDA has indicated that "it does not appear that the risk [of rhabdomyolysis] is greater with Crestor than with other marketed statins", but has mandated that a warning about this side effect, as well as a kidney toxicity warning, be added to the product label.[5] (https://academickids.com:443/encyclopedia/index.php/Rosuvastatin#fn_FDAlabel)
References
- AstraZeneca PLC (2005). Annual Report and Form 20-F, Information 2004 (http://www.astrazeneca.com/sites/7/imagebank/typearticleparam511562/astrazeneca-2004-annual-report.pdf). Information from print version.
- AstraZeneca PLC (2004). Annual Report and Form 20-F, 2003 (http://www.astrazeneca.com/sites/7/imagebank/typearticleparam503063/AstraZeneca%20Annual%20Report%202003.pdf). Retrieved 2005-03-20.
- McTaggart, F.; Buckett, L.; Davidson, R.; Holdgate, G.; McCormick, A.; Schneck, D.; Smith, G.; and Warwick, M. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy- 3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001;87(5supp1);28-32. PMID 11256847.
Notes
- Template:Anb AstraZeneca PLC (June 17, 2003). Core Data Sheet, Crestor Tablets. (http://www.crestor.info/gUserFiles/CRESTOR_CDS_10_40_mg_FINAL_170603.pdf) Retrieved 2005-03-20. NOTE: this is provider-oriented information and should not be used without the supervision of a physician.
- Template:Anb ibid.
- Template:Anb The Food and Drug Administration (August 12, 2003). FDA Approves New Drug for Lowering Cholesterol (http://www.fda.gov/bbs/topics/ANSWERS/2003/ANS01247.html). Press Release. Retrieved 2005-03-20.
- Template:Anb Horton, Richard (October 25, 2003). The statin wars: why AstraZeneca must retreat. (http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-49V58KN-1&_coverDate=10%2F25%2F2003&_alid=258485295&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=4886&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=ce2d0b8bd4d33f68f98df533b594ebc2) The Lancet 362(9393), 1341. PMID 14585629. Retrieved 2005-03-20. No author is listed with the online abstract; full-text is not available free online.
McKillop, Tom (November 1, 2003). The statin wars. (http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-49WMK9W-11&_coverDate=11%2F01%2F2003&_alid=258487424&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=4886&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=c03c924e107b06c6e03c9ebf377bbc9e) The Lancet 362 (9394), 1498. PMID 14602449. Full-text is not available free online. - Template:Anb Rosuvastatin Calcium (marketed as Crestor) Information (http://www.fda.gov/cder/drug/infopage/rosuvastatin/default.htm) (March 14, 2005). FDA Alert (03/2005). Retrieved 2005-03-20. This page is subject to change; the date reflects the last revision date.
External links
- Crestor web site (http://www.crestor.com/)
- Rosuvastatin Information (http://www.rosuvastatininformation.com), site run by AstraZeneca
- Crestor/Rosuvastatin Fact Sheet (http://www.fact-sheets.com/health/drugs-medications/crestor/)
- Rosuvastatin (Crestor) Information (http://www.emedicinehealth.com/articles/53027-1.asp)
- Possible Crestor Side Effects (http://www.emedicinehealth.com/articles/53027-6.asp)
FDA documents index
2005
- 11 March 2005: Letter from FDA to Public Citizen (http://www.fda.gov/cder/drug/infopage/rosuvastatin/crestor_CP.pdf) informing of the denial of Public Citizen's 4 March 2004 Citizen's Petition
- 8 March 2005: Letter from FDA to AstraZeneca (http://www.fda.gov/cder/warn/2005/Crestor_letter.pdf) regarding "false or misleading claims regarding the superiority of Crestor".
- 2 March 2005: FDA Public Health Advisory on Crestor (rosuvastatin) (http://www.fda.gov/cder/drug/advisory/crestor_3_2005.htm), patient and healthcare provider information updated
- 2 March 2005: Letter from FDA to AstraZeneca (http://www.fda.gov/cder/foi/appletter/2005/21366s005ltr.pdf) mandating changes to prescription labeling
2004
- 21 December 2004: Letter from FDA to AstraZeneca (http://www.fda.gov/cder/warn/2004/12779.pdf) regarding "false or misleading safety claims" in a print ad
- 4 November 2004: FDA Docket listing 2004P-0113 (http://www.fda.gov/ohrms/dockets/dailys/04/nov04/110404/110404.htm#04P0113), regarding Public Citizen's Citizens' Petition of 4 March 2004
- 15 September 2004: FDA Docket listing 2004P-0113 (http://www.fda.gov/ohrms/dockets/dailys/04/sep04/091504/091504.htm#04P0113), regarding Public Citizen's Citizens' Petition of 4 March 2004
- 1 September 2004: Letter from FDA to Public Citizen (http://www.fda.gov/ohrms/dockets/dailys/04/sep04/091504/04p-0113-let00001-vol1.pdf) indicating that Public Citizen's 4 March 2004 Citizen's Petition is still under consideration.
- 4 June 2004: FDA Docket listing 2004P-0113 (http://www.fda.gov/ohrms/dockets/dailys/04/June04/060404/060404.htm#04P0113), regarding Public Citizen's Citizens' Petition of 4 March 2004
- 18 May 2004: Letter from Public Citizen to FDA (http://www.fda.gov/ohrms/dockets/dailys/04/June04/060404/04p-0113-c00001-vol1.pdf), update to Citizen's Petition of 4 March 2004
- 6 March 2004: FDA Docket listing 2004P-0113 (http://www.fda.gov/ohrms/dockets/dailys/04/mar04/030504/030504.htm#04P0113), regarding Public Citizen's Citizens' Petition of 4 March 2004
- 5 March 2004: Letter from FDA to Public Citizen (http://www.fda.gov/ohrms/dockets/dailys/04/mar04/030504/04p-0113-ack0001-vol1.pdf) acknowledging receipt of Citizen's Petition.
- 4 March 2004: Letter from Public Citizen to FDA (http://www.fda.gov/ohrms/dockets/dailys/04/mar04/030504/04p-0113-cp0001-vol1.pdf) petitioning for the immediate removal of Crestor from the market
2003
- 12 August 2003: Letter from FDA to AstraZeneca (http://www.fda.gov/cder/foi/appletter/2003/21366ltr.pdf), approval letter
- 9 July 2003: Presentations to the Endocrinologoc (sic) and Metabolic Drugs Advisory Committee (http://www.fda.gov/ohrms/dockets/ac/03/slides/3968s1.htm)