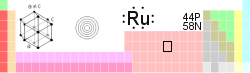
Ruthenium
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ruthenium, Ru, 44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 8, 5, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density, Hardness | 12370 kg/m3, 6.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Silvery white metallic Missing image Ru,44.jpg | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic weight | 101.07 amu | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 130 (178) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 126 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| van der Waals radius | no data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr]4d75s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| e- 's per energy level | 2, 8, 18, 15, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states (Oxide) | 2, 3, 4, 6, 8 (mildly acidic) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Hexagonal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State of matter | Solid (__) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2607 K (4233 ?F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4423 K (7502 ?F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 8.17 ×10-6 m3/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 595 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 24 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | 1.4 Pa at 2523 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 5970 m/s at 293.15 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.2 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 238 J/(kg*K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical conductivity | 13.7 106/(m·ohm) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 117 W/(m*K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1st ionization potential | 710.2 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd ionization potential | 1620 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd ionization potential | 2747 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ruthenium is a chemical element in the periodic table that has the symbol Ru and atomic number 44. A rare transition metal of the platinum group, ruthenium is found associated with platinum ores and used as a catalyst in some platinum alloys.
| Contents |
Notable characteristics
A polyvalent hard white metal, ruthenium is a member of the platinum group, has four crystal modifications and does not tarnish at normal temperatures, but does oxidize explosively. Ruthenium dissolves in fused alkalis, is not attacked by acids but is attacked by halogens at high temperatures and by hydroxides. Small amounts of ruthenium can increase the hardness of platinum and palladium. The corrosion resistance of titanium is increased markedly by the addition of a small amount of ruthenium.
This metal can be plated either through electrodeposition or by thermal decomposition methods. One ruthenium-molybdenum alloy has been found to be superconductive at 10.6 K. The oxidation states of ruthenium range from +1 to +8, and -2 is known, though oxidation states of +2, +3, and +4 are most common.
Applications
Due to its highly effective ability to harden platinum and palladium, ruthenium is used in Pt and Pd alloys to make severe wear resistance electrical contacts.
- 0.1% ruthenium is added to titanium to improve its corrosion resistance a hundredfold.
Ruthenium is also a versatile catalyst: Hydrogen sulfide can be split by light by using an aqueous suspension of CdS particles loaded with ruthenium dioxide. This may be useful in the removal of H2S from oil refineries and from other industrial processes.
Organometallic ruthenium carbene and allenylidene complexes have recently been found as highly efficient catalysts for olefin metathesis with important applications in organic and pharmaceutical chemistry.
Recently, large metallo-organic complexes of ruthenium have been found to exhibit anti-tumor activity and the first of a new group of anti-cancer medicine are now in the stage of clinical trials.
Some ruthenium complexes absorb light throughout the visible spectrum and are being actively researched in various, potential, solar energy technologies.
History
Ruthenium (Latin Ruthenia meaning "Russia") was discovered and isolated by Karl Klaus in 1844. Klaus showed that ruthenium oxide contained a new metal and obtained 6 grams of ruthenium from the part of crude platinum that is insoluble in aqua regia.
J?Berzelius and Gottfried Osann nearly discovered ruthenium in 1827. The men examined residues that were left after dissolving crude platinum from the Ural Mountains in aqua regia. Berzelius did not find any unusual metals, but Osann thought he found three new metals and named one of them ruthenium.
It is also possible that Polish chemist Jedrzej Sniadecki isolated element 44 (which he called vestium) from platinum ores in 1807. However his work was never confirmed and he later withdrew his discovery claim.
Occurrence
This element is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario and in pyroxinite deposits in South Africa.
This metal is commercially isolated through a complex chemical process in which hydrogen is used to reduce ammonium ruthenium chloride yielding a powder. The powder is then consolidated by powder metallurgy techniques or by argon-arc welding.
It is also possible to extract Ruthenium from burned-out nuclear fuel, which contains a few percent of Ruthenium. Ruthenium produced in such a way contains radioactive isotopes with half-lives of up to 373.59 days and is therefore stored at least 30 years in a secured area, before it can be sold.
Compounds
Ruthenium compounds are often similar in properties to those of osmium and exhibit at least eight oxidation states, but the +2, +3, and +4 states are the most common.
Isotopes
Naturally occurring ruthenium is composed of seven isotopes. The most stable radioisotopes are Ru-106 with a half-life of 373.59 days, Ru-103 with a half-life of 39.26 days and Ru-97 with a half-life of 2.9 days.
Fifteen other radioisotopes have been characterized with atomic weights ranging from 89.93 amu (Ru-90) to 114.928 (Ru-115). Most of these have half-lifes that are less than five minutes except Ru-95 (half-life: 1.643 hours) and Ru-105 (half-life: 4.44 h).
The primary decay mode before the most abundant isotope, Ru-102, is electron capture and the primary mode after is beta emission. The primary decay product before Ru-102 is technetium and the primary mode after is rhodium.
Organometallic chemistry
It is quite easy to form compounds with carbon ruthenium bonds, these compounds tend to be darker and react more quickly than the osmium compounds. Recently Prof Tony Hill and his co-workers have been making compounds of ruthenium in which a boron atom binds to the metal atom.
The most easy to make organometallic ruthenium compound is [RuHCl(CO)(PPh3)3]. Note if you every use this compound that it has two forms (yellow and pink). These forms are identical once they are dissolved but different in the solid state.
Precautions
The compound ruthenium tetroxide, RuO4, similar to osmium tetroxide, is highly toxic and may explode. Ruthenium plays no biological role but does strongly stain human skin, may be carcinogenic and bio-accumulates in bone.
References
- Los Alamos National Laboratory – Ruthenium (http://periodic.lanl.gov/elements/44.html)