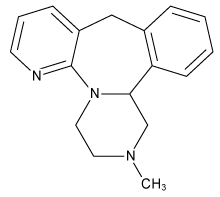
Mirtazapine
|
|
|
1,2,3,4,10,14b-hexahydro-2-methylpyrazino[2,1-a] pyrido [2,3-c] benzazepine | |
| Chemical formula | C17H19N3 |
| Molecular weight | 265.36 |
| Bioavailability | 50% |
| Metabolism | Liver |
| Elimination half life (females) | 37 hours |
| Elimination half life (males) | 26 hours |
| Excretion | |
| Pregnancy category | C |
| Delivery |
15mg, 30mg, and 45mg tablets |
Mirtazapine is a prescription antidepressant introduced by Organon in 1996, and marketed under the tradename Remeron® (also: Zispin®, Avanza®, Norset®, Remergil®). It is used primarily for the treatment of clinical depression in adults. It has a tetracyclic chemical structure and is a white to creamy white crystalline powder, distributed in tablets of 15mg, 30mg, and 45mg.
Mirtazapine is chemically unrelated to other antidepressants. It is thought to work by blocking presynaptic alpha-2 adrenergic receptors that normally inhibit the release of the neurotransmitters norepinephrine and serotonin, thereby increasing active levels in the synapse. Mirtazapine also blocks post-synaptic 5-HT2 and 5-HT3 receptors—an action which is thought to enhance serotonergic neurotransmission while causing a low incidence of side effects.
The side effects that do occur are thought to be primarily related to the blockage of histamine receptors, which decreases with higher dosages. These side effects may include:
- Drowsiness, especially at lower doses
- Dizziness
- Anxiousness
- Confusion
- Increased weight and appetite
- Dry mouth
- Constipation
- Upset stomach
- Vomiting
- Vivid, Unusual, and often times frightening dreams or nightmares