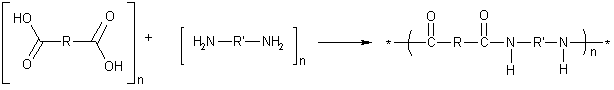Polymer
|
|
A polymer is a generic term used to describe a substantially long molecule. This long molecule consists of structural units and repeating units strung together through chemical bonds. The process of converting these units to a polymer is called polymerization. These units consist of monomers, which are typically small molecules of low molecular weight.
The monomers can be identical, or they can have one or more substituted chemical groups. These differences between monomers can affect properties such as solubility, flexibility, or strength. In proteins, these differences can give the polymer the ability to preferentially adopt one conformation over another, as opposed to adopting a random coil (see self-assembly). Although most polymers are organic (based on carbon chains), there are also inorganic polymers, mainly based on a silicon backbone.
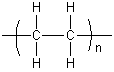
The term polymer covers a large, diverse group of molecules, including substances from proteins to high-strength kevlar fibres. A key feature that distinguishes polymers from other large molecules is the repetition of units of atoms (monomers) in their chains. This occurs during polymerization, in which many monomer molecules link to each other. For example, the formation of polyethene (also called polyethylene) involves thousands of ethene molecules bonding together to form a chain of repeating -CH2- units:
Polymers are often named in terms of their monomer units, for example polyethylene is represented by:
Because polymers are distinguished by their constituent monomers, polymer chains within a substance are often not of equal length. This is unlike other molecules in which every atom is acounted for, each molecule having a set molecular mass. Differing chain lengths occur because polymer chains terminate during polymerization after random intervals of chain lengthening (propagation).
Proteins are polymers of amino acids. From a dozen to some hundred of the (about) twenty different monomers form the chain, the sequence of monomers determining the shape and activity of the final protein. But there are active regions, surrounded by, as is believed now (Aug 2003), structural regions, whose sole role is to expose the active region(s) (there may be more than one on a given protein). So the absolute sequence of amino acids is not important, as long as the active regions are expressed (being accessible from the outside) properly. Also, whereas the formation of polyethylene occurs spontaneously given the right conditions, the manufacture of biopolymers such as proteins and nucleic acids requires the help of catalysts (substances that facilitate or accelerate reactions.) Since the 1950s, catalysts have also revolutionised the development of synthetic polymers. By allowing more careful control over polymerization reactions, polymers with new properties, such as the ability to emit coloured light, have been manufactured.
The well characterization of a polymer requires several parameters which need to be described. This is because a polymer actually consists of a distribution of chains of varying lengths, and each chain consists of monomer residues which affect its properties. Some of these parameters are described below.
| Contents |
Physical properties of polymers
Physical properties of polymers include degree of polymerization and molar mass distribution.
Branching
During the propagation of polymer chains, branching can occur. In radical polymerization, this is when a chain curls back and bonds to an earlier part of the chain. When this curl breaks, it leaves small chains sprouting from the main carbon backbone. Branched carbon chains cannot line up as close to each other as unbranched chains can. This causes less contact between atoms of different chains, and fewer opportunities for induced or permanent dipoles to occur. A low density results from the chains being further apart. Lower melting points and tensile strengths are evident, because the intermolecular bonds are weaker and require less energy to break.
Stereoregularity
Stereoregularity or tacticity describes the isomeric arrangement of functional groups on the backbone of carbon chains. Isotactic chains are defined as having substituent groups aligned in one direction. This enables them to line up close to each other, creating crystalline areas and resulting in highly rigid polymers.
In contrast, atactic chains have randomly aligned substituent groups. The chains do not fit together well and the intermolecular forces are low. This leads to a low density and tensile strength, but a high degree of flexibility.
Syndiotactic substituent groups alternate regularly in opposite directions. because of this regularity, syndiotactic chains can position themselves close to each, though not as close as isotactic polymers. Syndiotactic polymers have better impact strength than isotactic polymers because of the higher flexibility resulting from their weaker intermolecular forces.
Constitution of polymers
Copolymerization
Copolymerization is polymerization with two or more different monomers. Already mentioned are the twenty amino acid monomers that make up protein chains. Copolymerization of different monomers can result in varied properties of polymers, just as different amino acids result in different shapes of proteins. For example, copolymerising ethene with small amounts of hex-1-ene is one way to form linear low density polyethene (LLDPE) (See Polyethylene). The C4 branches resulting from the hexene lower the density and prevent such large crystalline regions within the polymer as in HDPE. This means that LLDPE can withstand strong tearing forces whilst remaining flexible.
The following image shows a specific type of copolymerization called a step-growth polymerization, or condensation polymerization. In this particular polymerization a small molecule is released upon polymerization. In the following reaction scheme, water is given off and nylon is formed. The type of nylon (name and properties) are governed by the R and R' groups in the monomers used.
Chemical properties of polymers
Intermolecular forces
The attractive forces between polymer chains play a large part in determining a polymer's properties. Because polymer chains are so long, these interchain forces are amplified far beyond the attractions between conventional molecules. Also, longer chains are more amorphous (randomly oriented). Polymers can be visualised as tangled spaghetti chains - pulling any one spaghetti strand out is a lot harder the more tangled the chains are. These stronger forces typically result in high tensile strength and melting points.
The intermolecular forces in polymers are determined by dipoles in the monomer units. Polymers containing amide groups can form hydrogen bonds between adjacent chains; the positive hydrogen atoms in N-H groups of one chain are strongly attracted to the oxygen atoms in C=O groups on another. These strong hydrogen bonds result in, for example, the high tensile strength and melting point of kevlar. Polyesters have dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogens in H-C groups. Dipole bonding is not as strong as hydrogen bonding, so ethene's melting point and strength are lower than kevlar's, but polyesters have greater flexibility.
Ethene, however, has no permanent dipole. The attractive forces between polyethylene chains arise from weak van der Waals forces. Molecules can be thought of as being surrounded by a cloud of negative electrons. As two polymer chains approach, their electron clouds repel one another. This has the effect of lowering the electron density on one side of a polymer chain, creating a slight positive dipole on this side. This charge is enough to actually attract the second polymer chain. Van der Waals forces are quite weak, however, so polyethene melts at low temperatures.
Polymer characterization
A variety of laboratory techniques are used to determine the properties of polymers. Techniques such as wide angle xray scattering, small angle xray scattering, and small angle neutron scattering are used to determine the crystalline structure of polymers. Gel permeation chromatography is used to determine the number average molecular weight, weight average molecular weight, and polydispersity. FTIR is used to determine composition. Thermal properties such as the glass transition temperature and melting point can be determined by differential scanning calorimetry and dynamic mechanical analysis. Thermal degradation followed by analysis of the fragments is one more technique for determining the possible structure of the polymer.
Polymer known as polymer substrate is used for everyday banknotes in Australia and New Zealand, and is also used in commemorative notes in other countries.
See also: Polymerization -- Biopolymer -- Condensation polymer -- Addition polymer -- Synthetic polymer -- Glass transition temperature -- Important publications in polymer chemistry
External links
- Polymer dictionary (http://www.borealisgroup.com/public/dictionary/Dictionary.jsp)
- Responsive Biopolymers for Drug Delivery and Imaging (http://www.vivamer.com/)
- Polymer Chemistry Hypertext, Educational resource (http://web.umr.edu/~wlf/)
- Polymer Chemistry Innovations (http://www.polychemistry.com/)
- Materials for Organic devices (http://www.odcad.com/html/organicdevice_appearance1.HTM)
- The Macrogalleria - a cyberwonderland of polymer fun! (http://www.pslc.ws/macrog/index.htm)
ca:Polímer cs:Polymer de:Polymer et:Polümeer es:Polímero eo:Polimero fr:Polymčre id:Polimer he:פולימר hu:Polimer ms:Polimer nl:Polymeer ja:重合体 pl:Polimer pt:Polímero fi:Polymeeri su:polimér