Lemon battery
|
|
- This experiment does not work as currently desribed. A single lemon battery cannot light an incandescent bulb. Four lemon batteries wired in series, however, can light a red LED (light-emitting diode).
- Using a magnesium strip instead of zinc should approximately double the current produced from the lemon cell (240 mA with zinc to about 400 mA with magnesium) and slightly increase the voltage (.97 V with zinc to 1.6 V with magnesium). These numbers of course depend upon your lemons.
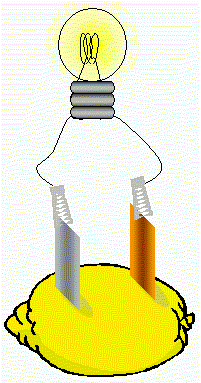
| Contents |
Apparatus
- A large lemon
- A strip of zinc; cleaned with sandpaper
- A strip of copper; cleaned with sandpaper
- A low voltage mes bulb (flashlight bulb) rated at 1.5 V
- Insulated copper wire with crocodile clips soldered onto the end.
Method
Assemble the apparatus as in the diagram on the right. Push the pieces of metal firmly into the lemon to act as electrodes. They must not touch one another. Clip the crocodile clips onto the electrodes and wire them to a bulb. Provided the bulb is sufficiently low voltage it will light. A battery powered digital clock sometimes works where a bulb will not because bulbs draw larger currents than digital displays.
This cell provides about 1V. If a large voltage is required they can be connected together in series. Connect the copper electrode of one cell to the zinc electrode of another cell.
How it Works
At the Anode
Both oxidation and reduction occur.
A lemon cell is more complicated than a simple cell.
Zinc is oxidised
- Zn → Zn2+ + 2 e-
Hydrogen is reduced
- 2H++ 2e- → H2
At the Cathode
Hydrogen is also reduced at this electrode.
- 2H++ 2e- → H2
