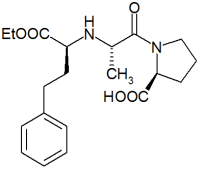
Enalapril
|
|
|
(S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl] -l-alanyl]-l-proline | |
|
Missing image | |
|
Data below refers to enalapril unless indicated | |
| Empirical formula | C20H28N2O5 |
| Molecular weight | 376.4 |
| Bioavailability (Oral) | 60% |
| Metabolism | hepatic (to enalaprilat) |
| Elimination half life (Oral) | 11 hours (enalaprilat) |
| Excretion | renal |
| Pregnancy category | D (Australia) |
Enalapril is an angiotensin converting enzyme (ACE) inhibitor used in the treatment of hypertension and some types of chronic heart failure. Enalapril was the first member of the group of ACE inhibitors known as the dicarboxylate-containing ACE inhibitors. It is marketed by Merck & Co. (Merck, Sharp & Dohme) under the trade names, Renitec® and Vasotec®.
Development
Enalapril was developed by researchers at Merck & Co. as part of their efforts to develop novel treatments for hypertension by modulating the renin-angiotensin-aldosterone (RAS) system.
The success of Squibb in developing the first inhibitor, captopril, provided a major impetus for Merck's research laboratories to develop a competing product. Captopril was not without its problems, however, as it was believed (and shown to be true) that the sulfhydryl-moeity of captoril was responsible for such adverse effects as metallic taste.
Enalaprilat
Enalaprilat, the first dicarboxylate-containing ACE inhibitor, was developed partly to overcome these limitations of captopril. The sulfhydryl-moeity was replaced by a carboxylate-moeity, but additional modifications were required in its structure-based design to achieve a similar potency to captopril.
Enalaprilat itself, however, was not without its problems. The consequence of the structural modifications was that it proved to be have unfavourable ionisation characteristics to allow sufficient potency for oral administration (in tablets). Thus enalaprilat was only suitable for intravenous administration. This was overcome by the researchers at Merck by the esterification of enalaprilat with ethanol to produce enalapril. As a prodrug, enalapril is metabolised in vivo to the active form enalaprilat by various esterases.
A prototype for others
Most importantly, perhaps, the QSAR-based modifications in structure serendipitously led to an improved understanding of the structure of ACE which aided in the development of subsequent carboxylate-containing ACE inhibitors.nn:Enalapril