Carbohydrate
|
|
Carbohydrates (literally hydrates of carbon) are chemical compounds that act as the primary biological means of storing or consuming energy, other forms being fat and protein. Relatively complex carbohydrates are known as polysaccharides. Carbohydrates are naturally produced by plants and animals. Sugars and starches are carbohydrates. A more precise definition of carbohydrates could be: Carbohydrates are polyhydroxyaldehydes, or polyhydroxyketones, and their derivatives.
| Contents |
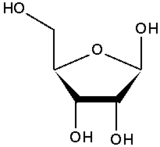
Structure
D-glucose.png
D-fructose.png
Pure carbohydrates contain carbon, hydrogen, and oxygen atoms, in a 1:2:1 molar ratio, giving the general formula CnH2nOn. However, many important carbohydrates deviate from this, such as deoxyribose. Sometimes compounds containing other elements are also counted as carbohydrates (e.g. chitin, which contains nitrogen).
The simplest carbohydrates are monosaccharides, which are small straight-chain aldehydes and ketones with many hydroxyl groups added, usually one on each carbon except the functional group. Other carbohydrates are composed of monosaccharide units, and break down under hydrolysis. These may be classified as disaccharides, oligosaccharides, or polysaccharides, depending on whether they have two, several, or many monosaccharide units.
Monosaccharides
Monosaccharides may be divided into aldoses, which have an aldehyde group on the first carbon atom, and ketoses, which typically have a ketone group on the second. They may also be divided into trioses, tetroses, pentoses, hexoses, and so forth, depending on how many carbon atoms they contain. For instance, glucose is an aldohexose, fructose a ketohexose, and ribose an aldopentose.
Further, each carbon atom that supports a hydroxyl group (except for the first and last) is optically active, allowing a number of different carbohydrates with the same basic structure. For instance, galactose is an aldohexose, but has different properties from glucose because the atoms are arranged differently.
The straight-chain structure described here is only one of the forms a monosaccharide may take. The aldehyde or ketone group may react with a hydroxyl group on a different carbon atom to form a hemiacetal or hemiketal, in which case there is an oxygen bridge between the two carbon atoms, forming a heterocyclic ring. Rings with five and six atoms are called furanose and pyranose forms, and exist in equilibrium with the straight-chain form.
It should be noted that the ring form has one more optically active carbon than the straight-chain form, and so has both an alpha and a beta form, which interconvert in equilibrium. However, the carbohydrate may further react with an alcohol to form an acetal or ketal, in which case the two forms become distinct. This is the basic type of link between the monosaccharide units of larger carbohydrates.
Disaccharides
Disaccharides are composed of two monosaccharide units bound together by a covalent glycosidic bond. The binding between the two sugars results in the loss of a hydrogen atom (H) from one molecule and a hydroxyl group (OH) from the other.
The most common disaccharides are sucrose (cane or beet sugar - made from one glucose and one fructose), lactose (milk sugar - made from one glucose and one galactose) and maltose (made of two glucoses). The formula of these disaccharides is C12H22O11.
Oligosaccharides and Polysaccharides
Oligosaccharides and Polysaccharides are composed of longer chains of monosaccharide units bound together by glycosidic bonds. The distinction between the two is based upon the number of monosaccharide units present in the chain. Oligosaccharides typically contain between 3 and 9 monosaccharide units and polysaccharides contain greater than 10 monosaccharide units. Definitions of how large a carbohydrate must be to fall into each category vary however.
Oligosaccharides are found as common form of protein posttranslational modification. Polysaccharides represent an important class of biological polymer. Examples include starch, cellulose and chitin.
Nutrition

Strictly speaking, carbohydrates are not necessary for human nutrition because proteins can be converted to carbohydrates—the traditional diet of some peoples consists of nearly zero percent carbohydrate, and they are perfectly healthy. However, carbohydrates require less water to digest than proteins or fats, and are an important source of energy.
Problems have been cited for the long term effects of a no-carbohydrate diet. These include reduced athletic performance, possible brain damage, and nephrotoxicity. The brain can only utilize carbohydrates for energy, and protein may not supply enough in many cases. The increase in protein means that more ammonia groups need to be removed from the blood.
Catabolism
There are three metabolic pathways of carbohydrate catabolism:
See also
External links
- IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Carbohydrate Nomenclature (http://www.chem.qmw.ac.uk/iupac/2carb/)
- Carbohydrates detailed (http://www.cem.msu.edu/~reusch/VirtualText/carbhyd.htm)
- Carbohydrates Overview (http://www.carbohydrate-counter.org/about-carbohydrates.php)
- Carbohydrates and Glycosylation - The Virtual Library of Biochemistry and Cell Biology (http://www.biochemweb.org/carbohydrates.shtml)ca:Hidrat de carboni
da:Kulhydrat de:Kohlenhydrate es:Hidrato de carbono eo:Karbonhidrato fr:Glucide id:Karbohidrat he:פחמימות lv:Ogļhidrāti lt:Angliavandenis nl:Koolhydraat ja:炭水化物 pl:Węglowodan pt:Carboidrato su:Karbohidrat fi:Hiilihydraatti sv:Kolhydrat zh:糖类