Trinitrotoluene
|
|
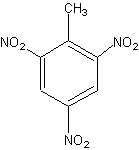
Trinitrotoluene (TNT, or Trotyl) is a pale yellow crystalline aromatic hydrocarbon compound that melts at 354 K (178 °F, 81 °C). Trinitrotoluene is an explosive chemical and a part of many explosive mixtures, such as when mixed with ammonium nitrate to form amatol. It is prepared by the nitration of toluene (C6H5CH3), it has a chemical formula of C6H2(NO2)3CH3, and IUPAC name 2,4,6-trinitromethylbenzene.
In its refined form, trinitrotoluene is very stable, and unlike nitroglycerin, it is insensitive to friction, blows or jarring. This means that it must be set off by a detonator. It does not react with metals or absorb water, and so unlike dynamite can be safely stored for many years. It is however readily acted upon by alkalis to form unstable compounds that are very sensitive to heat and impact.
Amounts of TNT are used as units of energy, based on a specific combustion energy of TNT of 4.184 MJ/kg (or one calorie—specifically a thermochemical calorie—per milligram). Hence 1 kt TNT = 4.184 TJ, 1 Mt TNT = 4.184 PJ. Thus with the mass–energy equivalence at 90 PJ/kg, 1 Mt TNT corresponds to 47 grams. Note that chemical explosives release less energy per kilogram than everyday household products like fat (38 MJ/kg) or sugar (17 MJ/kg); they do, however, release their combustion energy much more rapidly. One reason for their low power is that they contain their oxidant as well as the fuel — an explosive does not use atmospheric oxygen. The density of pure TNT (without any additives like sawdust or aluminum) is 1.654 g/cm³.
Toxicity
Some military testing grounds are contaminated with TNT. Wastewater from munitions programs including contamination of surface and subsurface waters may be colored pink as the result of TNT and RDX contamination. Such contamination, called pinkwater, may be difficult and expensive to remediate.
TNT is quite toxic. It can also be absorbed through the skin, and will cause irritation and bright yellow staining. During the First World War, munition workers who handled the chemical found that their skin turned bright yellow, which led to the nickname for such workers, canary girls or canaries, and ginger hair would turn green. A 1916 Government inquiry on female workers at Woolwich Arsenal found that 37% had severe pains due to loss of appetite, nausea and constipation, 25% suffered from dermatitis, and 34% experienced changes in menstruation. Before respirators and protective grease applied to the skin were introduced, about 100 workers died from the disease.
People who are exposed to TNT over a prolonged period tend to experience anemia and abnormal liver functions. Blood and liver effects, spleen enlargement and other harmful effects on the immune system have also been found in animals that ingested or breathed trinitrotoluene. There is evidence that TNT adversely affects male fertility, and TNT is listed as a possible human carcinogen. Consumption of TNT produces black urine.
History
TNT was first made in 1863 by a German chemist Joseph Wilbrand, but its potential was not seen for several years, mainly because it was so hard to detonate and because it was less powerful than other explosives. Amongst its advantages, however, are that it can be safely melted using steam or hot water and so poured molten into shell cases. It is also so insensitive that, for example, in 1910 it was exempted from the British 1875 Explosives Act from actually being considered as an explosive for the purposes of manufacture and storage.
The German armed forces adopted it as an artillery shell filling in 1902, and the British gradually started using it as replacement for lyddite in 1907. A particular advantage that it gave the German Navy in the First World War was that their TNT-filled armour piercing shells would detonate after they had penetrated the armour of British capital ships, whereas the British lyddite filled shells tended to explode as soon as they struck the German armour and thus expend their energy outside of the ship.
Because of the insatiable demand for it during the war, it was frequently mixed with 40-80% ammonium nitrate, producing an explosive called amatol. This was nearly as powerful as TNT, but suffered from the slight disadvantage that ammonium nitrate is hygroscopic.
See also
da:Trotyl de:Trinitrotoluol es:TNT fa:تیانتی fr:Trinitrotoluène lv:TNT nl:Trinitrotolueen ja:トリニトロトルエン pl:Trotyl pt:TNT ro:Trinitrotoluen fi:TNT sv:Trotyl zh:三硝基甲苯