Venlafaxine
|
|
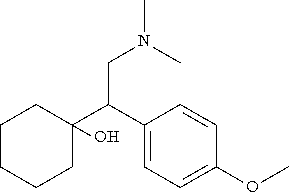
Venlafaxine hydrochloride is a prescription antidepressant first introduced by Wyeth in 1993, and marketed under the trade names EffexorŽ for tablets and Effexor XRŽ for extended-release capsules. EfexorŽ and Efexor XRŽ / EfexorŽ Depot are alternate trade name spellings used in some countries. Since venlafaxine is under patent, a generic is not available (as of 2005). It is used primarily for the treatment of depression, generalized anxiety disorder, and social anxiety disorder in adults. The chemical structure of venlafaxine is designated (R/S)-1-[2-(dimethylamino)-1-(4 methoxyphenyl)ethyl] cyclohexanol hydrochloride or (ą)-1-[a [α- (dimethylamino)methyl] p-methoxybenzyl] cyclohexanol hydrochloride and it has the empirical formula of C17H27NO2 · HCl. It is a white to off-white crystalline solid, distributed in pentagon-shaped peach-colored tablets of 25 mg, 37.5 mg, 50 mg, 75 mg, and 100 mg. There is also an extended-release version distributed in capsules of 37.5 mg (gray/peach), 75 mg (peach), and 150 mg (brown).
Venlafaxine is chemically unrelated to other antidepressants, but is sometimes categorized as a serotonin-norepinephrine reuptake inhibitor (SNRI). It works by blocking the transporter "reuptake" proteins for key neurotransmitters affecting mood, thereby leaving more active in the synapse. At low dosages, venlafaxine blocks serotonin reuptake, similarly to a selective serotonin reuptake inhibitor (SSRI). At medium dosages, venlafaxine blocks the reuptake of norepinephrine as well as serotonin. At high dosages, venlafaxine blocks the reuptake of serotonin and norepinephrine, and has a weak affinity for blocking dopamine reuptake.
Prescribed dosages are typically in the range of 75–225 mg per day, but higher dosages are sometimes used for the treatment of severe or treatment-resistant depression. Because of its relatively short half-life of 4 hours, venlafaxine should be administered in divided dosages throughout the day. The extended release version eliminates this problem and has largely replaced the original in use. Steady-state concentrations of Venlafaxine and its metabolite are attained in the blood within 3 days. Therapeutic effects are usually achieved within 3–4 weeks.
EffexorPill.jpg
Venlafaxine is somewhat notorious for its severe withdrawal symptoms upon sudden discontinuation. (The recommended discontinuation is a drop of 35 mg a week, and sudden stops are usually advised only in emergencies.) Wyeth-Ayerst refers to these severe withdrawal symptoms in its literature by the euphemism "severe discontinuation syndrome". These have a tendency to be stronger than the withdrawal effects of many antidepressants, but are similar in nature to those of tricyclic antidepressants and SSRIs such as Paroxetine (PaxilŽ). These effects may include headache, nausea, fatigue, and "brain shivers". Rarer withdrawal symptoms include shaking legs, dizziness and dysphoria. "Brain shivers" have been described as electric-like shocks in the brain causing pounding headaches and disorientation, increasing over time before abating. Although "Brain shivers" aren't exactly painful they can severe enough to be disabling. Antidepressant withdrawal effects do not indicate addiction, but are rather the results of the brain attempting to reach neurochemical stability. These can be minimalized or avoided by tapering off of the medication over a period of weeks. However, studies by Wyeth-Ayerst and others have reported very rare cases of withdrawal symptoms severe enough to require permanent use. In some of these cases, successful discontinuation was eventually achieved by the addition of fluoxetine, which was later discontinued itself without difficulty.
As with most anti-depressants, lack of sexual desire can be a very disturbing side-effect for some persons. Venlafaxine can raise blood pressure at high doses, so it is usually not the drug of choice for persons with high blood pressure. Venlafaxine should not be used in children. Caution should also be used in those with a seizure disorder.
Venlafaxine is an effective antidepressant for many persons; however, it seems to be especially effective for those with treatment-resistant depression. Some of these persons have taken two or three antidepressants prior to venlafaxine with no relief. Venlafaxine has also been found to reduce the severity of 'hot-flashes' in menopausal women [1] (http://www.mayoclinic.com/invoke.cfm?id=HQ01409).
Common side effects include:
- Nausea
- Dizziness
- Sleepiness
- Sexual Dysfunction
- Sweating
- Dry mouth
- Vivid Dreams
- Increase of Blood Pressure
Less-Common side-effects include:
- Gas or stomach pain
- Abnormal vision
- Nervousness
- Insomnia
- Loss of appetite
- Constipation
- Confusion/agitation
- Tremor
- Drowsiness
- Vertigo