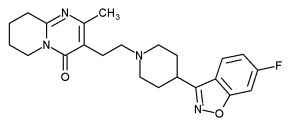
Risperidone
|
|
Risperidone (Belivon®, Rispen®, Risperdal®) is an atypical antipsychotic medication. It is most often used to treat delusional psychosis (including schizophrenia), but is also used in the treatment of bipolar disorder (as well as other members of the atypical antipsychotic family), and has shown some success in treating symptoms of Asperger's Syndrome and autism. Generally lower doses are used for autism spectrum disorders than are used for schizophrenia, although risperidone not yet been officially approved by the FDA for the treatment of autism and Asperger's.
| Contents |
Side effects
Risperidone can potentially cause tardive dyskinesia (TD), extrapyramidal symptoms (EPS), and neuroleptic malignant syndrome (NMS). Common side effects include: nausea, anxiety, dizziness, insomnia, low blood pressure, muscle stiffness, muscle pain, sedation, tremors, and weight gain. It has also been known to cause sexual dysfunction, and occasionally lactation in both genders, through effects on hormones such as estrogen and prolactin.
Pharmacology
Risperidone is a very strong dopamine blocker (antagonist); i.e., it inhibits functioning of dopamine receptors.
It reaches peak plasma levels quickly regardless of whether it is administered as a liquid or pills. The strong dopamine-blocking reaction is known to make some people feel nauseated if they do things that normally trigger the dopamine response, such as eat a pleasing meal or experience orgasm. Risperidone is metabolised fairly quickly so this potential for nausea subsides usually in two to three hours.
Current forms
Risperidone is available in the form of ordinary tablets, fast-dissolving tablets, and solution taken orally, and in the form of a long-lasting injection, known as Risperdal Consta. The latter is especially important, due to the high percentage of mentally ill persons (especially with schizophrenia) who are unable or unwilling to take daily medication orally because of their impaired ability to distinguish between reality and fantasy.
Risperdal is manufactured by Johnson & Johnson (NYSE: JNJ), which also makes Risperdal Consta in conjunction with Alkermes, Inc.
Chemistry
Risperidone is 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, C23H27FN4O2.
Safety risks
In 2004, the Committee for the Safety of Medicines (CSM) in the UK issued a warning that risperidone and another atypical antipsychotic, olanzapine, should not be given to elderly patients with dementia, because of an increased risk of stroke.
On July 24, 2004, Janssen Pharmaceutica Products LP, the subsidiary of Johnson & Johnson that manufactures Risperdal, admitted that it had minimized Risperdal's risks in its promotional material, risks that included strokes, diabetes and other potentially fatal complications. This disclosure followed a finding by the Food and Drug Administration that Janssen was continuing to make misleading claims that the medication was safer in treating mental illness than similar drugs. The Miami Herald also reported that several boys in Florida developed lactating breasts after taking Risperdal.
On June 17, 2005, government researchers presented a study that found a higher incidence of benign pituitary gland tumors in patients taking Risperidone. Lactation in men and children is a possible symptom of tumors in the pituitary gland. The findings do not conclude whether the tumors were caused by the drug or other factors.
External links
- http://www.healthsquare.com/newrx/RIS1382.HTM
- http://www.risperdal.com/
- Miami Herald: Maker of drug admits hiding its risks (http://www.miami.com/mld/miamiherald/9231611.htm?1c)zh:利培酮