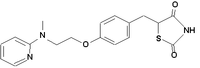
Pioglitazone
|
|
| 5-((4-(2-(5-ethyl-2-pyridinyl) ethoxy)phenyl)methyl)-,(+-)- 2,4-thiazolidinedione, | |
| CAS number 111025-46-8 | ATC code A10BG03 |
| Chemical formula | C19H20N2O3S |
| Molecular weight | 356.444 |
| Bioavailability | ? |
| Metabolism | ? |
| Elimination half-life | ? |
| Excretion | ? |
| Pregnancy category | ? |
| Legal status | ? |
| Routes of administration | ? |
In medicine and pharmacology, pioglitazone is a member of the drug class of the thiazolidinediones.
It is being marketed as Actos® by the pharmaceutical companies Takeda and Eli Lilly.
Like other thiazolidinediones, its mechanism of action is by activation the intracellular receptor class of the peroxisome proliferator-activated receptors (PPARs), specifically PPARγ.
Side-effects and contraindications
- See main article: thiazolidinedione
See also
External links
- MedlinePlus article (http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a699016.html) on pioglitazone.
- Diabetes monitor (http://www.diabetesmonitor.com/actos.htm) on Pioglitazone
- Actos / Pioglitazone Fact Sheet (http://www.fact-sheets.com/health/drugs-medications/actos/)