Nucleophile
|
|
In chemistry, a nucleophile (literally nucleus lover) is a reagent which is attracted to centres of positive charge. A nucleophile participates in a chemical reaction by donating electrons to a species known as an electrophile in order to form a chemical bond. Because nucleophiles donate electrons, they are by definition Lewis bases (see acid-base reaction theories). All molecules or ions with a free pair of electrons can act as nucleophiles, although negative ions (anions) are more potent than neutral reagents. Neutral nucleophilic reactions such as with alcohols, ROH, and water, HOH, are named solvolysis.
Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge on an element and displaces the group it is bonded to.
Nucleophilic is an adjective that describes the affinity of a nucleophile to the nuclei, while nucleophilicity or nucleophile strength refers to the nucleophilic character. Nucleophilicity is often used to compare an atom's relative affinity to another's.
The more basic the ions (high pKa) the more reactive they are as a nucleophile. Polarizability is also important in the determination of the nucleophilicity. Larger atoms contain more electrons; therefore distortion is easier so they are more nucleophilic. eg. I- is more nucleophilic than F-
| Contents |
Common examples
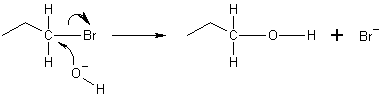
In the example below, the oxygen of the hydroxide ion donates an electron to bond with the carbon at the end of the bromopropane molecule. The bond between the carbon and the bromine then undergoes Heterolytic fission, with the bromine atom taking the pair of electrons and become the bromide ion Br-: