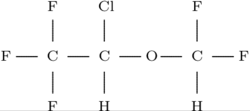
Isoflurane
|
|
Isoflurane (1-chloro-2,2,2-trifluoroethyl
difluoromethyl ether) is a halogenated ether used for inhalation anesthesia. Together with enflurane and halothane it replaced the flammable ethers used in the pioneer days of surgery. Its use is now starting to decline, being replaced with sevoflurane, desflurane and the intravenous anaesthetic propofol.
Isoflurane is always administered in conjunction with air and/or pure oxygen. Often nitrous oxide is also used. Although its physical properties means that anaesthesia can be induced more rapidly than with halothane, its pungency can irritate the respiratory system, negating this theoretical advantages conferred by its physical properties. It is usually used to maintain a state of general anesthesia that has been induced with another drug, such as thiopentone or propofol.
A major advantage of isoflurane is that it is no longer patented, and hence very economical to use.
It vaporizes readily, but is a liquid at room temperature. It is completely non-flammable.
Physical properties
| Molecular weight | 184.5 g/mol | ||
| Boiling point (at 1 atm): | 48.5 °C | ||
| Density (at 25 °C): | 1.496 g/mL | ||
| Vapor pressure: | 238 mmHg | 31.7 kPa | (at 20°C) |
| 295 mmHg | 39.3 kPa | (at 25°C) | |
| 367 mmHg | 48.9 kPa | (at 30°C) | |
| 450 mmHg | 60.0 kPa | (at 35°C) |